Genetic Syndromes and Genes Involved
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Successful Uterovaginal Anastomosis in an Unusual Presentation Of
JSAFOMS Successful Uterovaginal Anastomosis in an Unusual Presentation10.5005/jp-journals-10032-1056 of Congenital Absence of Cervix CASE REPORT Successful Uterovaginal Anastomosis in an Unusual Presentation of Congenital Absence of Cervix 1Nusrat Mahmud, 2Naushaba Tarannum Mahtab, 3TA Chowdhury, 4Anjan Kumar Deb ABSTRACT Source of support: Nil Cervical agenesis or dysgenesis (fragmentation, fibrous cord Conflict of interest: None and obstruction) is an extremely rare congenital anomaly. Conser vative surgical approach to these patients involves uterovaginal anastomosis, cervical canalization and cervical INTRODUCTION reconstruction. In failed conservative surgery, total hysterec- Primary amenorrhea is defined as absence of menstrua- tomy is the treatment of choice. We report what we believe to be the first successful end-to-end uterovaginal anastomosis of tion by the age of 14 years in the absence of secondary an unusual case of congenital cervical agenesis. A 25-year- sex characteristics or the absence of periods by the age of old female presented complaining of primary amenorrhea 16 years regardless of appearance of secondary sex and primary subfertility for the same duration. At laparoscopy, complete separation between the cervix and the body of the charac ters. In our last study, a series of total 108 cases uterus was found and hanging from surrounding supports. of primary amenorrhea were reviewed. It was found Both ovaries and fallopian tubes were anatomically positioned. that 69.4% were due Müllerian dysgenesis, 19.4% due to There was another muscular tissue of 2 cm in diameter at the gonadal dysgenesis, 2.7% male pseudohermaphroditism pouch of Douglas which was attached with lateral pelvic wall 13 by transverse cervical ligament. -

Cervical and Vaginal Agenesis: a Novel Anomaly
Cervical and Vaginal Agenesis: A Novel Anomaly Case Report Cervical and Vaginal Agenesis: A Novel Anomaly Irum Sohail1, Maria Habib 2 1Professor Obs/Gynae, 2Postgraduate Trainee, Wah Medical College, Wah Cantt Address of Correspondence: Dr. Maria Habib, Postgraduate Trainee, Wah Medical College, Wah Cantt Email: [email protected] Abstract Background: Cervical agenesis with vaginal agenesis is an extremely rare congenital anomaly. This mullerian anomaly occurs in 1 in 80,000-100,000 births. It is classified as type IB in the American Fertility Society Classification of mullerian anomalies. Case report: We report a case presented to POF Hospital, Wah cantt with primary amenorrhea and cyclic lower abdominal pain. She was diagnosed to have cervical agenesis associated with completely absent vagina. Conservative surgical approach to these patients involve uterovaginal anastomosis and cervical reconstruction. Creation of neovagina is necessary in these cases. Due to high failure rate and potential for complications, total hysterectomy with vaginoplasty is the treatment of choice by many authors. Conclusion: A thorough investigation of the patients with primary amenorrhea is necessary and total hysterectomy with vaginoplasty is feasible and should be considered as a first-line treatment option in cases of cervical and vaginal agenesis. Key words: Primary amenorrhea, Cervical agenesis, Vaginal agenesis, Hysterectomy. Introduction The female reproductive organs develop from the Society of Human Reproduction and Embryology fusion of the bilateral paramesonephric (Müllerian) (ESHRE)/European Society for Gynaecological ducts to form the uterus, cervix, and upper two- Endoscopy (ESGE) classification system of female thirds of the vagina.1 The lower third of the vagina genital anomalies is designed for clinical orientation develops from the sinovaginal bulbs of the and it is based on the anatomy of the female urogenital sinus.2 Mullerian duct anomalies (MDAs) genital tract. -

Uterine Conserving Surgery in a Case of Cervicovaginal Agenesis with Cloacal Malformation
International Journal of Reproduction, Contraception, Obstetrics and Gynecology Mishra V et al. Int J Reprod Contracept Obstet Gynecol. 2017 Mar;6(3):1144-1148 www.ijrcog.org pISSN 2320-1770 | eISSN 2320-1789 DOI: http://dx.doi.org/10.18203/2320-1770.ijrcog20170604 Case Report Uterine conserving surgery in a case of cervicovaginal agenesis with cloacal malformation Vineet Mishra1*, Suwa Ram Saini2, Priyankur Roy1, Rohina Aggarwal1, Ruchika Verneker1, Shaheen Hokabaj1 1Department of Obstetrics and Gynecology, IKDRC, Ahmedabad, Gujarat, India 2Department of Obstetrics and Gynecology, S. P. Medical College, Bikaner, Rajasthan, India Received: 30 December 2016 Accepted: 02 February 2017 *Correspondence: Dr. Vineet Mishra, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Cervico-vaginal agenesis (MRKHS) with normally formed uterus along with cloacal malformation is a very rare mullerian anomaly. We report a case, of a 13-year-old girl who was admitted at our tertiary care center with complaints of primary amenorrhea and cyclical lower abdominal pain for 3 months. Clinical examination and radiological investigations revealed complete cervico-vaginal agenesis with normal uterus with hematometra with horse shoe kidney. Vaginoplasty was done by McIndoe’s method with uterovaginal anastomosis and neocervix formation. Malecot’s catheter was inserted in uterine cavity. Vaginal mould was kept in the neovagina. Mould was removed after 10 days under anaesthesia and repeat hysteroscopy with insertion of a small piece of malecot’s catheter was performed under hysteroscopic guidance into the uterine cavity through neocervix and lower end fixed to the vagina. -

Pathophysiology, Diagnosis, and Management of Pediatric Ascites
INVITED REVIEW Pathophysiology, Diagnosis, and Management of Pediatric Ascites ÃMatthew J. Giefer, ÃKaren F. Murray, and yRichard B. Colletti ABSTRACT pressure of mesenteric capillaries is normally about 20 mmHg. The pediatric population has a number of unique considerations related to Intestinal lymph drains from regional lymphatics and ultimately the diagnosis and treatment of ascites. This review summarizes the physio- combines with hepatic lymph in the thoracic duct. Unlike the logic mechanisms for cirrhotic and noncirrhotic ascites and provides a sinusoidal endothelium, the mesenteric capillary membrane is comprehensive list of reported etiologies stratified by the patient’s age. relatively impermeable to albumin; the concentration of protein Characteristic findings on physical examination, diagnostic imaging, and in mesenteric lymph is only about one-fifth that of plasma, so there abdominal paracentesis are also reviewed, with particular attention to those is a significant osmotic gradient that promotes the return of inter- aspects that are unique to children. Medical and surgical treatments of stitial fluid into the capillary. In the normal adult, the flow of lymph ascites are discussed. Both prompt diagnosis and appropriate management of in the thoracic duct is about 800 to 1000 mL/day (3,4). ascites are required to avoid associated morbidity and mortality. Ascites from portal hypertension occurs when hydrostatic Key Words: diagnosis, etiology, management, pathophysiology, pediatric and osmotic pressures within hepatic and mesenteric capillaries ascites produce a net transfer of fluid from blood vessels to lymphatic vessels at a rate that exceeds the drainage capacity of the lym- (JPGN 2011;52: 503–513) phatics. It is not known whether ascitic fluid is formed predomi- nantly in the liver or in the mesentery. -

AMENORRHOEA Amenorrhoea Is the Absence of Menses in a Woman of Reproductive Age
AMENORRHOEA Amenorrhoea is the absence of menses in a woman of reproductive age. It can be primary or secondary. Secondary amenorrhoea is absence of periods for at least 3 months if the patient has previously had regular periods, and 6 months if she has previously had oligomenorrhoea. In contrast, oligomenorrhoea describes infrequent periods, with bleeds less than every 6 weeks but at least one bleed in 6 months. Aetiology of amenorrhea in adolescents (from Golden and Carlson) Oestrogen- Oestrogen- Type deficient replete Hypothalamic Eating disorders Immaturity of the HPO axis Exercise-induced amenorrhea Medication-induced amenorrhea Chronic illness Stress-induced amenorrhea Kallmann syndrome Pituitary Hyperprolactinemia Prolactinoma Craniopharyngioma Isolated gonadotropin deficiency Thyroid Hypothyroidism Hyperthyroidism Adrenal Congenital adrenal hyperplasia Cushing syndrome Ovarian Polycystic ovary syndrome Gonadal dysgenesis (Turner syndrome) Premature ovarian failure Ovarian tumour Chemotherapy, irradiation Uterine Pregnancy Androgen insensitivity Uterine adhesions (Asherman syndrome) Mullerian agenesis Cervical agenesis Vaginal Imperforate hymen Transverse vaginal septum Vaginal agenesis The recommendations for those who should be evaluated have recently been changed to those shown below. (adapted from Diaz et al) Indications for evaluation of an adolescent with primary amenorrhea 1. An adolescent who has not had menarche by age 15-16 years 2. An adolescent who has not had menarche and more than three years have elapsed since thelarche 3. An adolescent who has not had a menarche by age 13-14 years and no secondary sexual development 4. An adolescent who has not had menarche by age 14 years and: (i) there is a suspicion of an eating disorder or excessive exercise, or (ii) there are signs of hirsutism, or (iii) there is suspicion of genital outflow obstruction Pregnancy must always be excluded. -

Vaginal Agenesis: a Case Report*
Vaginal agenesis: A case report* By Reyalu T. Tan, MD; Sigrid A. Barinaga, MD, FPOGS; and Marie Janice S. Alcantara, MD, FPOGS Department of Obstetrics and Gynecology, Southern Philippine Medical Center ABSTRACT Congenital anomalies of the vagina are rare congenital anomalies. Women born with this anomaly present with collection of blood in the uterine cavity or hematometra and pelvic pain. Presented is a case of a 12-year old girl with hypogastric pain and primary amenorrhea complicated by vaginal agenesis. She was managed conservatively by creating a neovagina with the use of bipudendal flap or Modified Singapore flap. Management can be non-surgical or surgical but the management of congenital vaginal agenesis remains controversial. The decision to do a conservative surgical procedure or a hysterectomy depends on the clinical profile of the patient, the expertise of the surgeons, the extent of the anomaly, and its association to other congenital anomalies. Keywords: Vaginal Agenesis, Hematometra, Primary Amenorrhea, Modified Singapore flap INTRODUCTION congenital anomaly. The patient is an Elementary student, non-smoker, non-alcoholic beverage drinker, 2nd child of a evelopmental anomalies in mullerian ducts and G5P5 mother. urogenital sinus represent some of the most Two months prior to admission, the patient had Dinteresting disorders in Obstetrics and Gynecology. sudden onset of severe abdominal pain. Admitted at Normal development of the female reproductive system a local hospital and managed as a case of Ovarian New leads to differentiation of the reproductive structures. Growth with complication. At laparotomy, the patient Vaginal agenesis is the congenital absence of vagina was noted with hemoperitoneum (100 milliliter) with where there is failure of formation of the sinovaginal bulb the left fallopian tube enlarged to 5 x 9 centimeter with a which leads to outflow tract obstruction and infertility. -

Congenital Uterine Anomalies: the Role of Surgery Maria Carolina Fernandes Lamouroux Barroso M 2021
MESTRADO INTEGRADO EM MEDICINA Congenital uterine anomalies: the role of surgery Maria Carolina Fernandes Lamouroux Barroso M 2021 Congenital uterine anomalies: the role of surgery Dissertação de candidatura ao grau de Mestre em Medicina, submetida ao Instituto de Ciências Biomédicas Abel Salazar – Universidade do Porto Maria Carolina Fernandes Lamouroux Barroso Aluna do 6º ano profissionalizante de Mestrado Integrado em Medicina Afiliação: Instituto de Ciências Biomédicas Abel Salazar – Universidade do Porto Endereço: Rua de Jorge Viterbo Ferreira nº228, 4050-313 Porto Endereço eletrónico: [email protected]; [email protected] Orientador: Dra. Márcia Sofia Alves Caxide e Abreu Barreiro Diretora do Centro de Procriação Medicamente Assistida e do Banco Público de Gâmetas do Centro Materno-Infantil do Norte Assistente convidada, Instituto de Ciências Biomédicas Abel Salazar – Universidade do Porto. Afiliação: Instituto de Ciências Biomédicas Abel Salazar – Universidade do Porto Endereço: Largo da Maternidade de Júlio Dinis 45, 4050-651 Porto Endereço eletrónico: [email protected] Coorientador: Prof. Doutor Hélder Ferreira Coordenador da Unidade de Cirurgia Minimamente Invasiva e Endometriose do Centro Materno- Infantil do Norte Professor associado convidado, Instituto de Ciências Biomédicas Abel Salazar – Universidade do Porto. Afiliação: Instituto de Ciências Biomédicas Abel Salazar – Universidade do Porto Endereço: Rua Júlio Dinis 230, B-2, 9º Esq, Porto Endereço eletrónico: [email protected] Junho 2021 Porto, junho de 2021 ____________________________________ (Assinatura da estudante) ____________________________________ (Assinatura da orientadora) ____________________________________ (Assinatura do coorientador) ACKNOWLEDGEMENTS À Dra. Márcia Barreiro, ao Dr. Luís Castro e ao Prof. Doutor Hélder Ferreira, por toda a disponibilidade e empenho dedicado à realização deste trabalho. Aos meus pais, irmão e avós, pela participação que desde sempre tiveram na minha formação, e pelo carinho e apoio incondicional. -

Bicornuate Uterus with Unilateral Fibroid - Surgical Procedure Or LNG-IUS – a Conservative Approach in a Patient Who Opted LNG As Contraception
Jemds.com Case Report Bicornuate Uterus with Unilateral Fibroid - Surgical Procedure or LNG-IUS – A Conservative Approach in a Patient Who Opted LNG as Contraception Ankita Yadav1, Shashi Prateek2, Latika Chawla3, Shailja Sharma4, Deepti Choudhary5 1Department of Obstetrics and Gynaecology, AIIMS Rishikesh, Dehradun, Uttarakhand, India. 2Department of Obstetrics and Gynaecology, AIIMS Rishikesh, Dehradun, Uttarakhand, India. 3Department of Obstetrics and Gynaecology, AIIMS Rishikesh, Dehradun, Uttarakhand, India. 4Department of Obstetrics and Gynaecology, AIIMS Rishikesh, Dehradun, Uttarakhand, India. 5Department of Obstetrics and Gynaecology, AIIMS Rishikesh, Dehradun, Uttarakhand, India INTRODUCTION Bicornuate uterus with leiomyoma is rare. A 30 - year - old patient with bicornuate Corresponding Author: uterus with fibroid presented with abnormal - uterine - bleeding and was treated non Dr. Latika Chawla, - surgically with LNG - IUS. Uterine fibroids and AUB affect the quality of life and Department of Obstetrics and Gynaecology, AIIMS Rishikesh-249203, remain a leading indication for hysterectomy. In young women, uterine preservation Dehradun, Uttarakhand, approaches should be preferred as far as possible. India. Abnormalities in fusion or formation of Mullerian duct results in uterine E-mail: [email protected] structural and functional abnormalities.1 One of the Mullerian duct anomalies, bicornuate uterus, occurs due to incomplete fusion of utero-vaginal horns at the level DOI: 10.14260/jemds/2020/640 of fundus. Bicornuate uterus is the most common Mullerian duct anomaly (25 % of cases )2,3 and association of bicornuate uterus with leiomyoma is very rare and there How to Cite This Article: Yadav A, Prateek S, Chawla L, et al. - have been very few cases reported till now.4,5 A case of bicornuate uterus with Bicornuate uterus with unilateral fibroid- unilateral fibroid is being reported who presented with abnormal uterine bleeding surgical procedure or lng- ius (a and pelvic pain and was treated non-surgically with LNG - IUS. -

Beckwith's Syndrome) M
Postgrad Med J: first published as 10.1136/pgmj.46.533.162 on 1 March 1970. Downloaded from 162 Case reports VAN DER SLUYS VEER, J., CHOUFOER, J.C., QUERIDO, A., Lancet, i, 1416. VAN DER HEUL, R.O., HOLLANDER, C.F. & VAN RIJSSEL, ZOLLINGER, R.M. & ELLIOTT, D.W. (1959) Pancreatic T.G. (1964) Metastasizing islet cell tumour of the pancreas endocrine function and peptic ulceration. Gastroenter- associated with hypoglycaemia and carcinoid syndrome. ology, 37, 401. Macroglossia, abnormal umbilicus and hypoglycaemia (Beckwith's syndrome) M. W. MONCRIEFF* J. R. MANN M.A., B.M., M.R.C.P. M.B., M.R.C.P., D.C.H. Lecturer in Paediatrics, Registrar in Paediatrics, University of Birmingham Birmingham Children's Hospital A. R. GOLDSMITH G. W. CHANCE M.B.B.S. M.B., M.R.C.P., D.C.H. Registrar in Pathology, Senior Lecturer in Paediatrics, Birmingham Children's Hospital University ofBirmingham Introduction lobe and three had a dome-like elevation of the The syndrome of exomphalos, macroglossia, post- posterior part of the diaphragm. The six surviving natal somatic gigantism and severe hypoglycaemia children developed a characteristic facies with prog- copyright. in various combinations was first described in seven nathos, mid-facial under development and slight infants by Beckwith (1963) and Beckwith et al. exophthalmos, and a mid-line frontal ridge. Post- (1964). At necropsy the main features were cyto- natal somatic gigantism occurred in five of the six megaly of the foetal adrenal cortex, renal medullary survivors. A further seven cases with macroglossia dysplasia, and hyperplasia of the pancreas and and umbilical abnormality were reported by Shafer kidneys. -

Orphanet Report Series Rare Diseases Collection
Marche des Maladies Rares – Alliance Maladies Rares Orphanet Report Series Rare Diseases collection DecemberOctober 2013 2009 List of rare diseases and synonyms Listed in alphabetical order www.orpha.net 20102206 Rare diseases listed in alphabetical order ORPHA ORPHA ORPHA Disease name Disease name Disease name Number Number Number 289157 1-alpha-hydroxylase deficiency 309127 3-hydroxyacyl-CoA dehydrogenase 228384 5q14.3 microdeletion syndrome deficiency 293948 1p21.3 microdeletion syndrome 314655 5q31.3 microdeletion syndrome 939 3-hydroxyisobutyric aciduria 1606 1p36 deletion syndrome 228415 5q35 microduplication syndrome 2616 3M syndrome 250989 1q21.1 microdeletion syndrome 96125 6p subtelomeric deletion syndrome 2616 3-M syndrome 250994 1q21.1 microduplication syndrome 251046 6p22 microdeletion syndrome 293843 3MC syndrome 250999 1q41q42 microdeletion syndrome 96125 6p25 microdeletion syndrome 6 3-methylcrotonylglycinuria 250999 1q41-q42 microdeletion syndrome 99135 6-phosphogluconate dehydrogenase 67046 3-methylglutaconic aciduria type 1 deficiency 238769 1q44 microdeletion syndrome 111 3-methylglutaconic aciduria type 2 13 6-pyruvoyl-tetrahydropterin synthase 976 2,8 dihydroxyadenine urolithiasis deficiency 67047 3-methylglutaconic aciduria type 3 869 2A syndrome 75857 6q terminal deletion 67048 3-methylglutaconic aciduria type 4 79154 2-aminoadipic 2-oxoadipic aciduria 171829 6q16 deletion syndrome 66634 3-methylglutaconic aciduria type 5 19 2-hydroxyglutaric acidemia 251056 6q25 microdeletion syndrome 352328 3-methylglutaconic -
PDF WEETH LECTURE Early Oral Cancers and Precancers
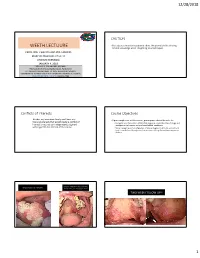
12/28/2018 CAUTION WEETH LECTUURE • Participants should be cautioned about the potential risks of using limited knowledge when integrating new techniques. EARLY ORAL CANCERS AND PRE-CANCERS MARY RIEPMA ROSS THEATER LINCOLN NEBRASKA JANUARY 4, 2019 DONALD M. COHEN DMD, MS, MBA PROFESSOR OF ORAL & MAXILOFACIAL PATHOLOGY ACTING CHAIR DEPARTMENT OF ORAL DIAGNOSTIC SCIENCS UNIVERSITY OF FLORIDA COLLEGE OF DENTISTRY GAINESVILLE, FLORIDA [email protected] 800-500-7585 Conflicts of Interests Course Objectives • Neither my immediate family nor I have any • Upon completion of this course, participants should be able to: financial interests that would create a conflict of • Recognize and formulate a differential diagnosis, understand the etiology and interest or restrict our independent judgment management of various oral and maxillofacial conditions. with regard to the content of this course. • Better recognize early mailignacies, improve diagnostic skills for oral soft and hard tissue lesions through practice sessions utilizing the audience response devices. GENERAL DENTIST TO ORAL SURGEON ORAL SURGOEN NOT TO WORRY PLEASE TAKE OUT TWO LOOSE TEETH TWO WEEK FOLLOW UP!! 1 12/28/2018 IDIOPATHIC LEUKOPLAKIA • FEATURES TO WORRY ABOUT • Occurrence in non-smoker • Thickened often corrugated appearance • Associated erythema • High risk location-horseshoe shaped area • ??pain • Multifocal or recurrent NON-SMOKERS LEUKOPLAKIA TONSILLAR CRYPT EPITHELIUM • Stratified squamous but basaloid so virus can • 5-8 times INCREASED risk of oral cancer invade