Brain Mapping Across 16 Autism Mouse Models Reveals a Spectrum of Functional Connectivity Subtypes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DELIA S. BALDASSARRI Professor New York University New York
Last updated: November, 2018 DELIA S. BALDASSARRI Professor New York University Phone: +1 212 998 8362 mail to: Department of Sociology Fax: +1 212 995 4140 295 Lafayette St. e-mail: [email protected] Puck Building, 4th Floor website: www.deliabaldassarri.org New York, NY, 10012 CURRENT AND PAST POSITIONS New York University 2016- Professor, Department of Sociology 2012- Associate Professor, Department of Sociology 2012- Affiliated Professor, Department of Politics 2012- Affiliated Professor, Management and Organizations, Stern School of Business 2013- Co-Director of the Center for Social and Political Behavior Princeton University 2007-2012 Assistant to Associate Professor (with tenure), Department of Sociology 2007-2012 Faculty Affiliate, Center for the Study of Democratic Politics 2008-2012 Faculty Affiliate, Office of Population Research 2009-2012 Advisory Committee, Center for the Study of Social Organization Other Affiliations 2017- Fellow of the Center for the Study of Economy and Society at Cornell University AY 2015-19 Senior Researcher, Dondena Centre for Research on Social Dynamics, Bocconi University, Milan AY 2012-13 Visiting Expert, Dondena Centre for Research on Social Dynamics, Bocconi University, Milan AY 2011-12 Visiting Scholar, Russell Sage Foundation, NYC Spring 2011 Visiting Scholar, Nuffield College, University of Oxford (Trinity Term) AY 2009-10 Visiting Scholar, Department of Sociology, New York University Spring 2009 Jemolo Fellow, Nuffield College, University of Oxford (Trinity Term) EDUCATION 2007 Ph.D. in Sociology (with distinction), Columbia University Dissertation: “Crosscutting Social Spheres? Political Polarization and the Social Roots of Pluralism.” Committee: Peter Bearman (chair), Harrison White, Duncan Watts, Andrew Gelman. 2006 Ph.D. in Sociology and Social Research, University of Trento, Italy Dissertation: “A Relational Approach to Collective Action: Analytical and Empirical Investigations.” Supervisor: Mario Diani. -

CV Elena Giacomelli- Europass 2 Copia
Curriculum Vitae Elena Giacomelli PERSONAL Elena Giacomelli INFORMATION Via Azzurra 14 40138 Bologna (BO) Italy ! (+39) 3493025187 [email protected] Sex Female | Date of birth 10/05/1992 | Nationality Italian POSITION APPLYING FOR settimanale l’INTERNAZIONALE EDUCATION AND TRAINING ! October 2016 - PhD Candidate - University of Bologna present - Social workers with asylum seekers; migration; ethnography; participant observation (supervisor: Professor Pina Lalli; email: [email protected] ) 12-14 December 4th International Conference Migrants and refugees in the Law 2018 Università Catolica de Murcia, Spain Speaker with the presentation “Ethnographic study of the reception project for asylum seekers and refugees in the Province of Trento, Italy. Reflections of the new professional figure: the "operatore d’accoglienza"" 17-19 September 1st Annual CESSMIR Conference: ‘Needs and Care Practices for Refugees and 2018 Migrants’ University of Ghent Speaker with a presentation on my research on ‘operatori d’accoglienza’ in Trentino province 10-14 September International Summer School in Ethnography 2018 Department of Sociology and Social research, University of Trento 6-8 September 2018 Summer School “Accogliere Come. Per una riforma del Sistema Nazionale di Accoglienza” - Europasilo Bologna, Italy 21-28 July 2018 Participation Amnesty International Summer Lab Lampedusa, Italy 2-6 July 2018 Summer School in Sociology of Migration - Centro Studi Medì Genova , Italy © European Union, 2002-2013 | http://europass.cedefop.europa.eu Page !1 / !4 Curriculum Vitae Elena Giacomelli 28-29 June 2018 Conference: Ripensare le Migrazioni forzate. Teorie, prassi, linguaggi e rappresentazioni - Escapes Laboratorio di studi critici sulle migrazioni forzate Milano, Italy January - June 2018 University of the Western Cape - EUROSA program - Erasmus Mundus Action 2 Study Abroad Program - granted of EUROSA scholarship (host supervisor: Prof. -

Curriculum Vitae of Maria Teresa Napoli
Curriculum Vitae of Maria Teresa Napoli EDUCATION Ph.D: Mechanical Engineering, March 2004 University of California, Santa Barbara, USA 1 Major in Dynamical Systems and Control 2 Advisor: Professor Bassam Bamieh Ph.D: Electrical Engineering, February 1999 Universita’ degli Studi di Padova, Padova, Italy 3 Major in Systems Theory and Control 4 Advisor: Professor Mauro Bisiacco M.S: Software Engineering, 1996. TecnoPadova, Padova Italy B. Tech: Electricall Engineering, July 1995 Universita’ degli Studi di Padova, Padova, Italy 5 Emphasis in Data Analysis and Control Theory 6 Advisor: Professor Mauro Bisiacco ACADEMIC EXPERIENCE Jan 2009 – present Assistant Project Scientist, Prof. Pennathur’s Nanolab, University of California, Santa Barbara Jan 2008 – Dec 2008 Postdoctoral Associate: University of California, Santa Barbara. Dec.2007 – Mar 2008 Lecturer appointment for the Master “Nano and Micro Electromechanical Systems” University of Trento, Italy. Dec.2006 – Mar 2007 Lecturer appointment for the Master “Nano and Micro Electromechanical Systems” University of Trento, Italy. May 2004 – Oct 2004 Postdoctoral Associate: University of California, Santa Barbara. Research: Implementation of observer-based sensing scheme for the estimation of displacement of electrostatically actuated MEMS devices. Fabrication and characterization of a novel tunable MEMS oscillator. 2001 – 2002 Postdoctoral Associate: Universita’ degli Studi di Padova, Italy. Research: Design of positioning system for HHD heads, Robust H∞ design. Sep 1998 – Mar 2004 Research Assistant: University of California, Santa Barbara. Research: Control and dynamical systems – modeling of multicantilever arrays, experimental characterization of electrostatically actuated microcantilevers, observer based design of decoupling controller, design of current measuring circuit. Sep 1998 – Jun 2002 Teaching Assistant: University of California, Santa Barbara. -

Yue Teng Address: Via Verdi 26, 38122 Trento, Italy Email: [email protected] Born on 30/04/1989, in Peking
Yue Teng Address: Via Verdi 26, 38122 Trento, Italy Email: [email protected] Born on 30/04/1989, in Peking Education 10/2014-ongoing, Ph.D. Programme in Development Economics · University of Trento (Università degli Studi di Trento), Italy University of Florence (Università degli Studi di Firenze), Italy · Dissertation: South-South Trade, Export Sophistication, and Structural Transformation: Empirical Studies on Developing Countries from 1995 to 2014 (in progress) · Supervisor: Professor Giuseppe Folloni 10/2012-10/2014, M.Sc. in International Economics · University of Tübingen (Eberhard Karls Universität Tübingen), Germany · Supervisor: Professor Jörg Baten 09/2011-08/2012, M.Sc. in Business Studies (Track in International Management) · University of Amsterdam (Universiteit van Amsterdam), the Netherlands · Supervisor: Dr Ilir Haxhi 09/2007-07/2011, Bachelor's Degree in International Economics and Trade · China Agricultural University, Peking, China · Supervisor: Professor Juan Qiao Academic Interests · Trade, Industrialisation, and Structural Transformation · Political Economy of Globalisation and Marxian Economics · Soviet Development Model and Soviet Economic History Working Papers · What Determines the Sophistication of Developing Countries' Northbound Exports and Southbound Exports? (Chapter 4 of Ph.D. dissertation) · The Illusion of South-South Trade (Chapter 2 of Ph.D. dissertation) · Life in the Periphery of Empire: An Anthropometric Assessment of Living Standard and Its 1 Ethnoregional Disparity in the Soviet Central Asian and -

UNIVERSITY of TRENTO Guide for International Students
UNIVERSITY OF TRENTO Guide for international students University of Trento The University of Trento is a dynamic, middle-sized, research-oriented university, featuring high quality teaching and research facilities, located in one of the most gorgeous natural environment in the world, the Dolomites, UNESCO World Heritage since 2009. The international positioning of the University of Trento is clearly outlined in international rankings: Trento has been confirmed among the very few Italian universities which have been ranked by QS World University and THE - Times Higher Education Rankings. The University of Trento has signed agreements with universities from all over the world that grant students and PhD students the possibility to further enrich their academic career by spending a study or research period abroad. The agreements relate to exchange programmes, double-degree and joint-degree programmes. The ECTS system allows the reciprocal recognition of exams taken abroad. Presentation video at www.international.unitn.it/unitrento Academic offer at UniTrento ACADEMIC UNDERGRADUATE GRADUATE COURSES POST GRADUATE STRUCTURE COURSES COURSES Centre for • Viticulture and Enology Agriculture, Food, Environment Centre for • Biomolecular Science and • Cellular and Molecular • Biomolecular Sciences1 Integrative Biology Technologies Biotechnology1 • Quantitative and Computational Biology1 Centre for Mind/ • Cognitive Science1 • Cognitive and Brain Brain Sciences Sciences1 Department of • Civil Engineering • Civil Engineering • Civil, Environmental and -

University of Trento, Italy
University of Trento, Italy Institutional details The University of Trento is located in one of the most gorgeous natural environments in the world, the Dolomites, and it combines strong links to the territory with a steady expansion of international outreach. UniTrento is widely recognized as a dynamic and innovative research and teaching institution with strong connections to industry and top-class services. National and international classifications consistently rank the University of Trento as one of the top Italian universities. In addition to traditional lectures, there is much emphasis on Presentation laboratory work and small seminar groups. UniTrento is a medium-sized university, where it is easy for students and teachers to interact and there is a continuous exchange of ideas across many different disciplines: in short, the virtuous dynamism of a small university. Furthermore, as a result of long-term international relations, UniTrento is a vibrant, multi- cultural university which fosters an enriching and stimulating learning environment. Video The University of Trento: a video introduction Acronym UniTrento Postal Address Via Calepina, 14 - 38122 Trento, Italy Website International UniTrento ID Erasmus Code I TRENTO01 Erasmus Charter for HE 29348-LA-1-2014-1-IT-E4AKA1-ECHE for 2014-2020 period Proposal Number 101014693 already approved for 2021-2027 period Students Staff Facts and Figures 17.000 enrolled students in 2019-2020 a.y. 710 professors and researchers 1.300 international students 750 administrative staff Social sciences -

Annual Report 2019
ANNUAL REPORT 2019 SAR Italy is a partnership between Italian higher education institutions and research centres and Scholars at Risk, an international network of higher education institutions aimed at fostering the promotion of academic freedom and protecting the fundamental rights of scholars across the world. In constituting SAR Italy, the governance structures of adhering institutions, as well as researchers, educators, students and administrative personnel send a strong message of solidarity to scholars and institutions that experience situations whereby their academic freedom is at stake, and their research, educational and ‘third mission’ activities are constrained. Coming together in SAR Italy, the adhering institutions commit to concretely contributing to the promotion and protection of academic freedom, alongside over 500 other higher education institutions in 40 countries in the world. Summary Launch of SAR Italy ...................................................................................................................... 3 Coordination and Networking ....................................................................................................... 4 SAR Italy Working Groups ........................................................................................................... 5 Sub-national Networks and Local Synergies ................................................................................ 6 Protection .................................................................................................................... -

Summer Fellowships: How to Apply
SUMMER FELLOWSHIPS: HOW TO APPLY The Giovanni Armenise Harvard Foundation provides funding to Summer Fellows to receive training and conduct basic biomedical research in a laboratory at Harvard Medical School (HMS) or an aliated hospital, from July 1 – August 30, 2019. The program is aimed at Italian university students who wish to explore the American research system and who will return to their home institution to complete their degree. Note: clinical research or clinical activities are not supported by this program. The Foundation receives over 100 applications and awards 10-15 fellowships per year. The mean Grade Point Average of students awarded last year was 29 (calculated with “30 e lode” valued at 30). This program is under the Patronage of the Italian Ministry of Education, University and Research. ELIGIBILITY Italian students enrolled in an Italian public or private university in a “Laurea Specialistica” (4th and 5th year) with a focus on biomedical research (e.g. Biology, Biotechnology, Neuroscience, etc.) or in the 4th, 5th, or 6th year of Medicine. Students expecting to graduate before July 2018 are not eligible. Previous research experience is strongly recommended. The Armenise Harvard Summer Fellowship is NOT compatible with having any other summer fellowship or position that requires research activity at the same time. The Summer Fellowship program demands a full-time commitment on your part. FELLOWSHIP PAYMENT Successful applicants will be awarded a fellowship payment of 2,700 Euros intended to help cover costs associated with travel to Boston, housing during the fellowship, health insurance, visa application fees, and incidentals. PAST SPONSORS Fellowships in the past have been sponsored by the Giovanni Armenise Harvard Foundation, Charles River Laboratories, Collegio Ghislieri, Collegio Nuovo, Friends of the Italian Cultural Center of Boston (FICCB), Fondazione CEUR, University of Catania, University of Pavia, Italian National Research Council (CNR), Humanitas University and CIBIO/University of Trento. -
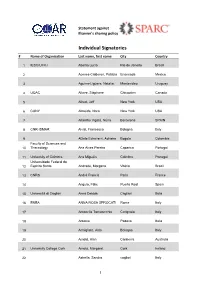
Elsevier Statement Signatories
Statement against Elsevier's sharing policy Individual Signatories #Name of Organisation Last name, first name City Country 1 IESC/UFRJ Abelha Lucia Rio de Janeiro Brasil 2 Aceves-Calderon, Patricia Ensenada Mexico 3 Aguirre-Ligüera, Natalia Montevideo Uruguay 4 UQAC Allaire, Stéphane Chicoutimi Canada 5 Allred, Jeff New York USA 6 CUNY Almeida, Nora New York USA 7 Altarriba Vigatà, Núria Barcelona SPAIN 8 CNR-ISMAR Alvisi, Francesca Bologna Italy 9 Alzate Echeverri, Adriana Bogota Colombia Faculty of Sciences and 10 Thecnology Ana Alves Pereira Caparica Portugal 11 University of Coimbra Ana Miguéis Coimbra Portugal Universidade Federal do 12 Espírito Santo Andrade, Morgana Vitória Brazil 13 CNRS André Francis Paris France 14 Angulo, Félix Puerto Real Spain 15 Università di Cagliari Anna Deidda Cagliari Italia 16 ENEA ANNA ROSA SPROCATI Rome Italy 17 Antonella Tomasicchio Cerignola Italy 18 Antonia Padova Italia 19 Armigliato, Aldo Bologna Italy 20 Arnold, Alan Canberra Australia 21 University College Cork Arnold, Margaret Cork Ireland 22 Astrella, Sandra cagliari Italy 1 #Name of Organisation Last name, first name City Country 23 Audrit, Stéphanie Liège Belgique 24 Austin, Roddy New York United States Universidade Federal do Rio 25 de Janeiro Azeredo, Antonio Rio de Janeiro Brazil Universitat Autònoma de 26 Barcelona Azorín, Cristina Barcelona Catalunya Repositorios Digitales, 27 MINCyT, Rep. Argentina Azrilevich, Paola Buenos Aires Argentina 28 CLACSO Babini, Dominique Buenos Aires Argentina 29 Babu Vivek Cennai India 30 Indiana University -

University of Trento. a European University
University of Trento. A European University. In Italy. THE UNIVERSITY OF TRENTO: great studying, green living 3 The University of Trento in a nutshell 4 History 5 International Rankings 6 EDUCATION AND RESEARCH great studying... 7 Departments and Centres 9 Research Centres 9 International programmes 11 Legal Studies area 11 International programmes 12 Biomolecular Sciences area 13 Cognitive Sciences area 15 Economics and Management area 17 Environmental Engineering area 21 ICT area 22 Industrial Engineering area 24 International Studies area 26 Mathematics area 28 Physics area 29 Social Studies area 30 PhD courses 31 International opportunities 32 Services and facilities for international students 34 SPORTS AND LEISURE TIME ...green living 38 2 University of Trento. A European University. In Italy. THE UNIVERSITY OF TRENTO: great studying, green living The University of Trento is located The University has remained small in Trentino, an Alpine region in in size, but great in quality, capable the north-eastern part of Italy, of playing a high-level, dominated by the majestic well-deserved, role at European Dolomites, numerous lakes, and international levels. forests and vineyards, offering Indeed, it has developed strong to its inhabitants and guests the European and international experience of an atmosphere networks of cooperation and journey suspended between the relationships, with a series of Alps and the Mediterranean. programmes and agreements with Its position on the Alpine- Universities in every continent. Mediterranean axis has always English is the teaching language encouraged an international of some Bachelor’s, numerous environment, which is clearly Master’s and Doctoral programmes reflected in the strongly and there are opportunities international-oriented approach to achieve double-degree of the University, whose qualifications with international internationalisation is indeed one partner Universities. -

Chesta Riccardo Emilio
Chesta Riccardo Emilio Via dei Roccettini, 9, San Domenico di Fiesole, Florence E-mail: [email protected] / [email protected] Mobile: +393403276145 EUI TIMES Profle https://me.eui.eu/riccardo-emilio-chesta/ COSMOS Profle: http://cosmos.sns.it/person/riccardo-emilio-chesta/ CURRENT POSITION 2013-2017 PhD Researcher in Social and Political Sciences Contentious Politics of Expertise. Experts, Activists and Grassroots Environmentalism European University Institute, Florence Supervisor: Prof. Donatella Della Porta (EUI – Scuola Normale Superiore, Florence) Co-Supervisor: Prof. Luigi Pellizzoni (University of Trieste/University of Pisa) EDUCATION 2013 Master of Research in Social and Political Sciences European University Institute, Florence 2011 Master of Research in Social Sciences École Normale Supérieure (Rue D'Ulm) École des Hautes Etudes en Sciences Sociales, Paris [17/20 (Trés Bien)] 2009 B.A. in Sociology, University of Trento [110/110 cum laude] SCIENTIFIC INTERESTS Sociological Theory & Epistemology Social Movements, Political Participation & Collective Action Sciences, Technology & Innovation Culture & Consumption PROFESSIONAL AND SCIENTIFIC EXPERIENCES 2017-2018 Research Assistant Department of Sociology and Political Sciences, Scuola Normale Superiore, Florence Project CRY_OUT – Critical Young Europeans Funded by Italian Ministry of Education and Research (MIUR) 2017 (March) Visiting Scholar at Urban Democracy Lab, Department of Sociology, New York University Liason: Prof. Gianpaolo Baiocchi (New York University-Gallatin -

Knowledges at Risk
Winter School 2018 Program and Speakers Spaces and Times of February 19 - 9.30-18.00 Opening session Globalization Francesca Gambarotto (University of Padova) Gilda Rota (University of Padova) Sixth Edition Jan Aart Scholte (University of Gothenburg) Lynn Mario Menezes de Souza (University of San Paolo) 15.00 – 18.00 Knowledges of Group work February 20 - 9.30-12.30 Human capital, skills, competences and the future. professionalization: wot of education? Jan Masschelein (University of Leuven) Professions of the future. Lynn Mario Menezes de Souza (University of San Paolo) Living in precarious times Asli Vatansever (SAR scholar, University of Padova) 14.30 – 18.00 Workshop at the University Botanical Garden Aula 19th – 23th February 2018 Emiciclo: Sharing Ignorances Telmo Pievani (University of Padova) c/o SPGI Department February 21 - 9.30-12.30 Innovation, technologies, post-human: clashing via del Santo 28 narratives? Luca De Biase (Il Sole 24 Ore) Padova Paolo Magaudda (University of Padova) Maria Sangiuliano (University of Venice) organized by David Burigana (University of Padova) Next Generation Global Studies Adriana Barsotti (professional journalist, Brazil) 14.30 – 17.30 SPGI, in cooperation with FISPPA Interactive workshop and students’ University of Padova, Italy presentations February 22 - 9.00-12.30 Feminist knowledges: from practice to rUniversityesearch. And of Padua [email protected] back? February 18 | Aula Nievo SPEAKERS Annabelle Sreberny (SAOS, London) Organized by Maxime Forest (SciencePo, Paris) http://www.spgi.unipd.it/corsi/winter-school-2018