Ecophysiology of Crassulacean Acid Metabolism (CAM)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Leaf Anatomy and C02 Recycling During Crassulacean Acid Metabolism in Twelve Epiphytic Species of Tillandsia (Bromeliaceae)
Int. J. Plant Sci. 154(1): 100-106. 1993. © 1993 by The University of Chicago. All rights reserved. 1058-5893/93/5401 -0010502.00 LEAF ANATOMY AND C02 RECYCLING DURING CRASSULACEAN ACID METABOLISM IN TWELVE EPIPHYTIC SPECIES OF TILLANDSIA (BROMELIACEAE) VALERIE S. LOESCHEN,* CRAIG E. MARTIN,' * MARIAN SMITH,t AND SUZANNE L. EDERf •Department of Botany, University of Kansas, Lawrence, Kansas 66045-2106; and t Department of Biological Sciences, Southern Illinois University, Edwardsville, Illinois 62026-1651 The relationship between leaf anatomy, specifically the percent of leaf volume occupied by water- storage parenchyma (hydrenchyma), and the contribution of respiratory C02 during Crassulacean acid metabolism (CAM) was investigated in 12 epiphytic species of Tillandsia. It has been postulated that the hydrenchyma, which contributes to C02 exchange through respiration only, may be causally related to the recently observed phenomenon of C02 recycling during CAM. Among the 12 species of Tillandsia, leaves of T. usneoides and T. bergeri exhibited 0% hydrenchyma, while the hydrenchyma in the other species ranged from 2.9% to 53% of leaf cross-sectional area. Diurnal malate fluctuation and nighttime atmospheric C02 uptake were measured in at least four individuals of each species. A significant excess of diurnal malate fluctuation as compared with atmospheric C02 absorbed overnight was observed only in T. schiedeana. This species had an intermediate proportion (30%) of hydrenchyma in its leaves. Results of this study do not support the hypothesis that C02 recycling during CAM may reflect respiratory contributions of C02 from the tissue hydrenchyma. Introduction tions continue through fixation of internally re• leased, respired C02 (Szarek et al. -

Spanish Moss and Ball Moss 1
FOR52 Spanish Moss and Ball Moss 1 Nancy P. Arny2 Spanish moss (Tillandsia usneoides) and ball Bromeliads moss (T. recurvata) are common elements of the Florida landscape. They are two of Florida's native Like almost all members of the Bromeliaceae, members of the Bromeliaceae, also known as the Spanish moss and ball moss are perennial herbs. This pineapple family. This family includes species as means they do not have permanent woody stems diverse as pineapples, Spanish moss and a above ground, but that individual plants persist for carnivorous relative native to Australia. Bromeliads years and will reproduce without human intervention. are members of the plant division Like many other bromeliads, these plants are Magnoliophyta--the flowering plants. While most epiphytes or "air plants". This indicates that they do Floridians are at least vaguely familiar with Spanish not require soil to root in, but can survive and thrive moss, many have never seen it flower and may be growing above the ground hanging on branches of surprised at the beauty of its delicate blossom. Of trees or other structures. They are not parasites. course, the fact that both Spanish moss and ball moss Without soil as a source of nutrients, these plants produce flowers is proof that they are not truly have evolved the capacity to make use of minerals mosses at all. dissolved in the water which flows across leaves and down branches. This fact sheet will help the reader to distinguish between the two common Tillandsias . It also Spanish moss plants appear to vary in mineral provides basic information on the biology and content and it has been proven that they gain a ecology of these fascinating plants and provides significant portion of their nutrients from stem recommendations for their management in the home run-off from the trees on which they are anchored. -

Network Scan Data
Selbyana 15: 132-149 CHECKLIST OF VENEZUELAN BROMELIACEAE WITH NOTES ON SPECIES DISTRIBUTION BY STATE AND LEVELS OF ENDEMISM BRUCE K. HOLST Missouri Botanical Garden, P.O. Box 299, St. Louis, Missouri 63166-0299, USA ABSTRACf. A checklist of the 24 genera and 364 native species ofBromeliaceae known from Venezuela is presented, including their occurrence by state and indications of which are endemic to the country. A comparison of the number of genera and species known from Mesoamerica (southern Mexico to Panama), Colombia, Venezuela, the Guianas (Guyana, Suriname, French Guiana), Ecuador, and Peru is presented, as well as a summary of the number of species and endemic species in each Venezuelan state. RESUMEN. Se presenta un listado de los 24 generos y 364 especies nativas de Bromeliaceae que se conocen de Venezuela, junto con sus distribuciones por estado y una indicaci6n cuales son endemicas a Venezuela. Se presenta tambien una comparaci6n del numero de los generos y especies de Mesoamerica (sur de Mexico a Panama), Colombia, Venezuela, las Guayanas (Guyana, Suriname, Guyana Francesa), Ecuador, y Peru, y un resumen del numero de especies y numero de especies endemicas de cada estado de Venezuela. INTRODUCTION Bromeliaceae (Smith 1971), and Revision of the Guayana Highland Bromeliaceae (Smith 1986). The checklist ofVenezuelan Bromeliaceae pre Several additional country records were reported sented below (Appendix 1) adds three genera in works by Smith and Read (1982), Luther (Brewcaria, Neoregelia, and Steyerbromelia) and (1984), Morillo (1986), and Oliva-Esteva and 71 species to the totals for the country since the Steyermark (1987). Author abbreviations used last summary of Venezuelan bromeliads in the in the checklist follow Brummit and Powell Flora de Venezuela series which contained 293 (1992). -

Australia Lacks Stem Succulents but Is It Depauperate in Plants With
Available online at www.sciencedirect.com ScienceDirect Australia lacks stem succulents but is it depauperate in plants with crassulacean acid metabolism (CAM)? 1,2 3 3 Joseph AM Holtum , Lillian P Hancock , Erika J Edwards , 4 5 6 Michael D Crisp , Darren M Crayn , Rowan Sage and 2 Klaus Winter In the flora of Australia, the driest vegetated continent, [1,2,3]. Crassulacean acid metabolism (CAM), a water- crassulacean acid metabolism (CAM), the most water-use use efficient form of photosynthesis typically associated efficient form of photosynthesis, is documented in only 0.6% of with leaf and stem succulence, also appears poorly repre- native species. Most are epiphytes and only seven terrestrial. sented in Australia. If 6% of vascular plants worldwide However, much of Australia is unsurveyed, and carbon isotope exhibit CAM [4], Australia should host 1300 CAM signature, commonly used to assess photosynthetic pathway species [5]. At present CAM has been documented in diversity, does not distinguish between plants with low-levels of only 120 named species (Table 1). Most are epiphytes, a CAM and C3 plants. We provide the first census of CAM for the mere seven are terrestrial. Australian flora and suggest that the real frequency of CAM in the flora is double that currently known, with the number of Ellenberg [2] suggested that rainfall in arid Australia is too terrestrial CAM species probably 10-fold greater. Still unpredictable to support the massive water-storing suc- unresolved is the question why the large stem-succulent life — culent life-form found amongst cacti, agaves and form is absent from the native Australian flora even though euphorbs. -

Guía Visual De Bromelias Presentes En Un Sector Del Parque Natural Chicaque
GUÍA VISUAL DE BROMELIAS PRESENTES EN UN SECTOR DEL PARQUE NATURAL CHICAQUE PRESENTADO POR: DIANA CAROLINA GUTIERREZ GONZALEZ ANDREA LISETH SALAMANCA BARRERA UNIVERSIDAD PEDAGÓGICA NACIONAL FACULTAD DE CIENCIA Y TECNOLOGÍA DEPARTAMENTO DE BIOLOGÍA BOGOTÁ 2015 1 GUÍA VISUAL DE BROMELIAS PRESENTES EN UN SECTOR DEL PARQUE NATURAL CHICAQUE PRESENTADO POR: DIANA CAROLINA GUTIERREZ GONZALEZ ANDREA LISETH SALAMANCA BARRERA Trabajo de grado presentado para optar al título de Licenciado en Biología DIRECTOR FRANCISCO MEDELLÍN CADENA UNIVERSIDAD PEDAGÓGICA NACIONAL FACULTAD DE CIENCIA Y TECNOLOGÍA DEPARTAMENTO DE BIOLOGÍA BOGOTÁ 2015 2 NOTA DE ACEPTACIÓN _____________________________ _____________________________ _____________________________ _____________________________ ________________________________________ DIRECTOR: FRANCISCO MEDELLÍN CADENA _______________________________________ JURADO ______________________________________ JURADO Diciembre, 2015 3 AGRADECIMIENTOS En primer lugar agradecemos al profesor Francisco Medellín Cadena por orientar este trabajo con sus asesorías , recomendaciones y conocimientos, también es necesario brindar un agradecimiento a la línea de investigación Ecología y Diversidad de los sistemas acuáticos de la región andina ( SARA) por aceptar este trabajo y proporcionarnos los instrumentos y bibliografía necesaria para su realización. En la realización de este trabajo también fue importante la ayuda de nuestro compañero Julián Romero y Wholfan Díaz que nos colaboraron con el préstamo del material necesario para obtener -

Temporal and Spatial Origin of Gesneriaceae in the New World Inferred from Plastid DNA Sequences
bs_bs_banner Botanical Journal of the Linnean Society, 2013, 171, 61–79. With 3 figures Temporal and spatial origin of Gesneriaceae in the New World inferred from plastid DNA sequences MATHIEU PERRET1*, ALAIN CHAUTEMS1, ANDRÉA ONOFRE DE ARAUJO2 and NICOLAS SALAMIN3,4 1Conservatoire et Jardin botaniques de la Ville de Genève, Ch. de l’Impératrice 1, CH-1292 Chambésy, Switzerland 2Centro de Ciências Naturais e Humanas, Universidade Federal do ABC, Rua Santa Adélia, 166, Bairro Bangu, Santo André, Brazil 3Department of Ecology and Evolution, University of Lausanne, CH-1015 Lausanne, Switzerland 4Swiss Institute of Bioinformatics, Quartier Sorge, CH-1015 Lausanne, Switzerland Received 15 December 2011; revised 3 July 2012; accepted for publication 18 August 2012 Gesneriaceae are represented in the New World (NW) by a major clade (c. 1000 species) currently recognized as subfamily Gesnerioideae. Radiation of this group occurred in all biomes of tropical America and was accompanied by extensive phenotypic and ecological diversification. Here we performed phylogenetic analyses using DNA sequences from three plastid loci to reconstruct the evolutionary history of Gesnerioideae and to investigate its relationship with other lineages of Gesneriaceae and Lamiales. Our molecular data confirm the inclusion of the South Pacific Coronanthereae and the Old World (OW) monotypic genus Titanotrichum in Gesnerioideae and the sister-group relationship of this subfamily to the rest of the OW Gesneriaceae. Calceolariaceae and the NW genera Peltanthera and Sanango appeared successively sister to Gesneriaceae, whereas Cubitanthus, which has been previously assigned to Gesneriaceae, is shown to be related to Linderniaceae. Based on molecular dating and biogeographical reconstruction analyses, we suggest that ancestors of Gesneriaceae originated in South America during the Late Cretaceous. -

Hoya Carnosa in a Subtropical Rain
UvA-DARE (Digital Academic Repository) Vascular epiphytes in Taiwan and their potential response to climate change Hsu, R.C.C. Publication date 2013 Link to publication Citation for published version (APA): Hsu, R. C. C. (2013). Vascular epiphytes in Taiwan and their potential response to climate change. General rights It is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), other than for strictly personal, individual use, unless the work is under an open content license (like Creative Commons). Disclaimer/Complaints regulations If you believe that digital publication of certain material infringes any of your rights or (privacy) interests, please let the Library know, stating your reasons. In case of a legitimate complaint, the Library will make the material inaccessible and/or remove it from the website. Please Ask the Library: https://uba.uva.nl/en/contact, or a letter to: Library of the University of Amsterdam, Secretariat, Singel 425, 1012 WP Amsterdam, The Netherlands. You will be contacted as soon as possible. UvA-DARE is a service provided by the library of the University of Amsterdam (https://dare.uva.nl) Download date:01 Oct 2021 Chapter 4 Canopy CO2 concentrations and crassulacean acid metabolism in Hoya carnosa in a subtropical rain forest in Taiwan: consideration of CO2 availability and the evolution of CAM in epiphytes Fushan is a subtropical rainforest with annual rainfall above 3.5 m. Although here the average daily humidity throughout the year typically approaches 95 %, our study indicated that the likely ecophysiological significance of CAM Hoya carnosa in H. -

Stapeliads, Morphology and Pollination, Welwitchia 5
Morfologija in opra{evanje stapelijevk Stapeliads, morphology and pollination Iztok Mulej Matija Strli~ Stapelijevke so so~nice s ~udovitimi cvetovi in Stapeliads are succulents with beautiful flowers vonjem, ki ga taki cvetovi ne zaslu`ijo. Raz{irjene with a smell that does not match their beauty at so ve~inoma v Afriki, dotikajo se Evrope, v Aziji all. Distributed mainly in Africa, a few species can pa imajo tudi precej predstavnikov. Cvetovi so also be found in Europe, and quite a few in Asia. nekaj posebnega, ne samo po bizarni lepoti am- Their flowers are unique, not only due to the pak tudi po zgradbi. Prav tako je tudi opra{itev bizarre beauty, but also due to the unusual repro- samosvoja, saj podobne ne najdemo nikjer drug- ductive structures. Even the pollination mecha- je v rastlinskem svetu. nism has no parallel in the plant kingdom. Klju~ne besede: Keywords: stapelijevke, Apocynaceae, Asclepiadoideae, Stapeliads, Apocynaceae, Asclepiadoideae, mor- morfologija, opra{evanje. fology, pollination. Stapeliads, which are stem succulents, belong World" is the title of the web pages of Jerry to the family Apocynaceae and subfamily As- Barad from New Jersey, USA. The title says clepiadoideae. Until recently, they were everything. The flowers have a beauty and placed into the Asclepiadaceae family. The colour that can only be compared with or- stem shapes are very similar in most genera, chids. And they also share another character- but when they bloom, the beauty of the flow- istic. The pollen mass is fused in a wax pollen ers is striking as well as their unpleasant sack - pollinium, which is transferred by pol- smell! "Stapeliads, Orchids of the Succulent linators to the style. -
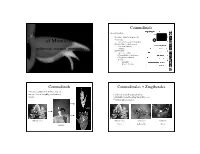
Diversity and Evolution of Monocots
Commelinids 4 main groups: Diversity and Evolution • Acorales - sister to all monocots • Alismatids of Monocots – inc. Aroids - jack in the pulpit • Lilioids (lilies, orchids, yams) – non-monophyletic . spiderworts, bananas, pineapples . – petaloid • Commelinids – Arecales – palms – Commelinales – spiderwort – Zingiberales –banana – Poales – pineapple – grasses & sedges Commelinids Commelinales + Zingiberales • theme: reduction of flower, loss of nectar, loss of zoophily, evolution of • 2 closely related tropical orders bracts • primarily nectar bearing but with losses • bracted inflorescences grass pickeral weed pickeral weed spiderwort heliconia nectar pollen only bracts rapatead bromeliad Commelinaceae - spiderwort Commelinaceae - spiderwort Family of small herbs with succulent stems, stems jointed; leaves sheathing. Family does not produce Inflorescence often bracted nectar, but showy flowers for insect pollen gathering. Rhoeo - Moses in a cradle Commelina erecta - Erect dayflower Tradescantia ohiensis - spiderwort Tradescantia ohiensis - spiderwort Commelinaceae - spiderwort Commelinaceae - spiderwort Flowers actinomorphic or • species rich in pantropics, CA 3 CO 3 A 6 G (3) zygomorphic especially Africa • floral diversity is enormous Commelina communis - day flower Tradescantia ohiensis - spiderwort Pontederiaceae - pickerel weed Pontederiaceae - pickerel weed Aquatic family of emergents or floaters. Pickerel weed has glossy heart-shaped leaves, Water hyacinth (Eichhornia) from superficially like Sagittaria but without net venation. -

The Quarterly Journal of the Florida Native Plant Society
Volume 27: Number 2 > Summer 2010 PalmettoThe Quarterly Journal of the Florida Native Plant Society Everglades Tree Islands ● Schizaea pennula ● Pricing the Priceless BOOK REVIEW Native Bromeliads of Florida Reviewed by Chuck McCartney Among plants adding to the bromeliads and orchids. Thus, the as well a mention of its distribution tropical ambience of much of Florida’s reader of Native Bromeliads of Florida outside Florida, plus other interesting natural landscape are members of could not ask for a more authoritative tidbits about the species. the plant family Bromeliaceae, the pair of writers on the subject. There is also a dichotomous key bromeliads. These are our so-called The book delineates Florida’s 18 to help distinguish among the three “air plants,” and they are the most native bromeliads, including the three native bromeliad genera (Catopsis, commonly seen and widespread that do not occur in the southern Guzmania and Tillandsia), with further group of epiphytes, or tree-growing end of the state – Tillandsia bartramii, keys to the three Catopsis species and plants, found in our state. the apparently endemic Tillandsia 14 tillandsias. The keys are written Bromeliaceae is sometimes called simulata, and Tillandsia x floridana, in language that’s fairly easy to under- the pineapple family because that a putative hybrid of T. bartramii and stand for the amateur, and there ground-growing species, Ananas T. fasciculata var. densispica. is a glossary in the back of the book comosus from Brazil, is the most It also discusses familiar South to help with any unfamiliar terms. familiar representative of the group. Florida species, such as the widespread But what makes this book equally But equally familiar to people who and beautiful Tillandsia fasciculata, informative is the introductory have traveled in the American South with its flame red flower spikes (even material. -

The Complete Chloroplast Genome Sequence of Asparagus (Asparagus Officinalis L.) and Its Phy- Logenetic Positon Within Asparagales
Central International Journal of Plant Biology & Research Bringing Excellence in Open Access Research Note *Corresponding author Wentao Sheng, Department of Biological Technology, Nanchang Normal University, Nanchang 330032, The Complete Chloroplast Jiangxi, China, Tel: 86-0791-87619332; Fax: 86-0791- 87619332; Email: Submitted: 14 September 2017 Genome Sequence of Accepted: 09 October 2017 Published: 10 October 2017 Asparagus (Asparagus ISSN: 2333-6668 Copyright © 2017 Sheng et al. officinalis L.) and its OPEN ACCESS Keywords Phylogenetic Positon within • Asparagus officinalis L • Chloroplast genome • Phylogenomic evolution Asparagales • Asparagales Wentao Sheng*, Xuewen Chai, Yousheng Rao, Xutang, Tu, and Shangguang Du Department of Biological Technology, Nanchang Normal University, China Abstract Asparagus (Asparagus officinalis L.) is a horticultural homology of medicine and food with health care. The entire chloroplast (cp) genome of asparagus was sequenced with Hiseq4000 platform. The complete cp genome maps a circular molecule of 156,699bp built with a quadripartite organization: two inverted repeats (IRs) of 26,531bp, separated by a large single copy (LSC) sequence of 84,999bp and a small single copy (SSC) sequence of 18,638bp. A total of 112 genes comprising of 78 protein-coding genes, 30 tRNAs and 4 rRNAs were successfully annotated, 17 of which included introns. The identity, number and GC content of asparagus cp genes were similar to those of other asparagus species genomes. Analysis revealed 81 simple sequence repeat (SSR) loci, most composed of A or T, contributing to a bias in base composition. A maximum likelihood phylogenomic evolution analysis showed that asparagus was closely related to Polygonatum cyrtonema that belonged to the genus Asparagales. -

Life Cycle Cost of Air Plant Green Roofs in Hot and Humid Climate
I J A B E R, Vol. 14, No. 10 (2016):Life 7167-7182 Cycle Cost of Air Plant Green Roofs in Hot and Humid Climate l 7167 LIFE CYCLE COST OF AIR PLANT GREEN ROOFS IN HOT AND HUMID CLIMATE Tachaya Sangkakool* and Kuaanan Techato2* Abstract: The benefitsof green roofshave beenrecognizedby many researchers worldwide.Green roofs have been wildly implemented in many countries due to the trend of green architecture, sustainable architecture and environmental friendly concept. The computational life cycle cost of air plant green roofs is classified into two parts. One is the initial investment, which compos- es of the cost of materials and installation process. Another is the cost of operation and main- tenance. This paper has investigated in the economics of green roofs by reviewing secondary data of extensive green roof and intensive green roofs and collecting experimental data of air plant green roofs. The investigation of life cycle cost of “Cotton Candy” air plant green roofs is around 140.21$/m2 and “Spanish moss” air plant green roofs is around 125.78 $/m2. Although the digit is lower than other types of green roofs, the benefit is almost the same. It was found from the research that life cycle cost of air plant green roof is less than other types of green roof. However, the benefits are not different from other type of the roof. Another strengthof air plant green roofs is shading to the roof of the building. These will extend the life cycle of the roof. The consideration of life cycle cost of air plant green roofs will be another tool using in making final decision.