Proton Beam Radiation Therapy – Commercial Medical Policy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Five Common Particles
The Five Common Particles The world around you consists of only three particles: protons, neutrons, and electrons. Protons and neutrons form the nuclei of atoms, and electrons glue everything together and create chemicals and materials. Along with the photon and the neutrino, these particles are essentially the only ones that exist in our solar system, because all the other subatomic particles have half-lives of typically 10-9 second or less, and vanish almost the instant they are created by nuclear reactions in the Sun, etc. Particles interact via the four fundamental forces of nature. Some basic properties of these forces are summarized below. (Other aspects of the fundamental forces are also discussed in the Summary of Particle Physics document on this web site.) Force Range Common Particles It Affects Conserved Quantity gravity infinite neutron, proton, electron, neutrino, photon mass-energy electromagnetic infinite proton, electron, photon charge -14 strong nuclear force ≈ 10 m neutron, proton baryon number -15 weak nuclear force ≈ 10 m neutron, proton, electron, neutrino lepton number Every particle in nature has specific values of all four of the conserved quantities associated with each force. The values for the five common particles are: Particle Rest Mass1 Charge2 Baryon # Lepton # proton 938.3 MeV/c2 +1 e +1 0 neutron 939.6 MeV/c2 0 +1 0 electron 0.511 MeV/c2 -1 e 0 +1 neutrino ≈ 1 eV/c2 0 0 +1 photon 0 eV/c2 0 0 0 1) MeV = mega-electron-volt = 106 eV. It is customary in particle physics to measure the mass of a particle in terms of how much energy it would represent if it were converted via E = mc2. -

Proton Stereotactic Body Radiation Therapy for Liver Metastases— Results of 5-Year Experience for 81 Hepatic Lesions
1760 Original Article Proton stereotactic body radiation therapy for liver metastases— results of 5-year experience for 81 hepatic lesions Alex R. Coffman1, Daniel C. Sufficool2, Joseph I. Kang1, Chung-Tsen Hsueh3, Sasha Swenson4, Patrick Q. McGee4, Gayathri Nagaraj3, Baldev Patyal1, Mark E. Reeves5, Jerry D. Slater1, Gary Y. Yang1 1Department of Radiation Oncology, Loma Linda University Medical Center, Loma Linda, CA, USA; 2Department of Radiation Oncology, Kettering Health Network, Kettering, OH, USA; 3Department of Medical Oncology, Loma Linda University Medical Center, Loma Linda, CA, USA; 4Loma Linda University School of Medicine, Loma Linda, CA, USA; 5Department of Surgical Oncology, Loma Linda University Medical Center, Loma Linda, CA, USA Contributions: (I) Conception and design: GY Yang; (II) Administrative support: B Patyal, JD Slater, GY Yang; (III) Provision of study materials or patients: CT Hsueh, G Nagaraj, ME Reeves; (IV) Collection and assembly of data: AR Coffman, GY Yang; (V) Data analysis and interpretation: AR Coffman, GY Yang; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Alex R. Coffman, MD. Department of Radiation Oncology, Loma Linda University Medical Center, 11234 Anderson Street, Suite B121, Loma Linda, CA 92354, USA. Email: [email protected]. Background: To report on our institutional experience using Proton stereotactic body radiation therapy (SBRT) for patients with liver metastases. Methods: All patients with liver metastases treated with Proton SBRT between September 2012 and December 2017 were retrospectively analyzed. Local control (LC) and overall survival (OS) were estimated using the Kaplan-Meier method calculated from the time of completion of Proton SBRT. LC was defined according to Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (version 1.1). -

An Analysis of Vertebral Body Growth After Proton Beam Therapy for Pediatric Cancer
cancers Article An Analysis of Vertebral Body Growth after Proton Beam Therapy for Pediatric Cancer Keiichiro Baba 1, Masashi Mizumoto 1,* , Yoshiko Oshiro 1,2, Shosei Shimizu 1 , Masatoshi Nakamura 1, Yuichi Hiroshima 1 , Takashi Iizumi 1, Takashi Saito 1, Haruko Numajiri 1, Kei Nakai 1 , Hitoshi Ishikawa 1,3, Toshiyuki Okumura 1, Kazushi Maruo 4 and Hideyuki Sakurai 1 1 Proton Medical Research Center, Department of Radiation Oncology, University of Tsukuba Hospital, Tsukuba, Ibaraki 305-8576, Japan; [email protected] (K.B.); [email protected] (Y.O.); [email protected] (S.S.); [email protected] (M.N.); [email protected] (Y.H.); [email protected] (T.I.); [email protected] (T.S.); [email protected] (H.N.); [email protected] (K.N.); [email protected] (H.I.); [email protected] (T.O.); [email protected] (H.S.) 2 Department of Radiation Oncology, Tsukuba Medical Center Hospital, Tsukuba, Ibaraki 305-8558, Japan 3 National Institutes for Quantum and Radiological Science and Technology, QST Hospital, Chiba 263-8555, Japan 4 Department of Clinical Trial and Clinical Epidemiology, Faculty of Medicine, University of Tsukuba, Tsukuba, Ibaraki 305-8575, Japan; [email protected] * Correspondence: [email protected]; Tel.: +81-29-853-7100; Fax: +81-29-853-7102 Simple Summary: Radiotherapy has a key role in treatment of pediatric cancer and has greatly improved survival in recent years. However, vertebrae are often included in the irradiated area, and this may affect growth after treatment. -

Targeted Radiotherapeutics from 'Bench-To-Bedside'
RadiochemistRy in switzeRland CHIMIA 2020, 74, No. 12 939 doi:10.2533/chimia.2020.939 Chimia 74 (2020) 939–945 © C. Müller, M. Béhé, S. Geistlich, N. P. van der Meulen, R. Schibli Targeted Radiotherapeutics from ‘Bench-to-Bedside’ Cristina Müllera, Martin Béhéa, Susanne Geistlicha, Nicholas P. van der Meulenab, and Roger Schibli*ac Abstract: The concept of targeted radionuclide therapy (TRT) is the accurate and efficient delivery of radiation to disseminated cancer lesions while minimizing damage to healthy tissue and organs. Critical aspects for success- ful development of novel radiopharmaceuticals for TRT are: i) the identification and characterization of suitable targets expressed on cancer cells; ii) the selection of chemical or biological molecules which exhibit high affin- ity and selectivity for the cancer cell-associated target; iii) the selection of a radionuclide with decay properties that suit the properties of the targeting molecule and the clinical purpose. The Center for Radiopharmaceutical Sciences (CRS) at the Paul Scherrer Institute in Switzerland is privileged to be situated close to unique infrastruc- ture for radionuclide production (high energy accelerators and a neutron source) and access to C/B-type labora- tories including preclinical, nuclear imaging equipment and Swissmedic-certified laboratories for the preparation of drug samples for human use. These favorable circumstances allow production of non-standard radionuclides, exploring their biochemical and pharmacological features and effects for tumor therapy and diagnosis, while investigating and characterizing new targeting structures and optimizing these aspects for translational research on radiopharmaceuticals. In close collaboration with various clinical partners in Switzerland, the most promising candidates are translated to clinics for ‘first-in-human’ studies. -
![Particle Accelerators and Detectors for Medical Diagnostics and Therapy Arxiv:1601.06820V1 [Physics.Med-Ph] 25 Jan 2016](https://docslib.b-cdn.net/cover/8515/particle-accelerators-and-detectors-for-medical-diagnostics-and-therapy-arxiv-1601-06820v1-physics-med-ph-25-jan-2016-558515.webp)
Particle Accelerators and Detectors for Medical Diagnostics and Therapy Arxiv:1601.06820V1 [Physics.Med-Ph] 25 Jan 2016
Particle Accelerators and Detectors for medical Diagnostics and Therapy Habilitationsschrift zur Erlangung der Venia docendi an der Philosophisch-naturwissenschaftlichen Fakult¨at der Universit¨atBern arXiv:1601.06820v1 [physics.med-ph] 25 Jan 2016 vorgelegt von Dr. Saverio Braccini Laboratorium f¨urHochenenergiephysik L'aspetto pi`uentusiasmante della scienza `eche essa incoraggia l'uomo a insistere nei suoi sogni. Guglielmo Marconi Preface This Habilitation is based on selected publications, which represent my major sci- entific contributions as an experimental physicist to the field of particle accelerators and detectors applied to medical diagnostics and therapy. They are reprinted in Part II of this work to be considered for the Habilitation and they cover original achievements and relevant aspects for the present and future of medical applications of particle physics. The text reported in Part I is aimed at putting my scientific work into its con- text and perspective, to comment on recent developments and, in particular, on my contributions to the advances in accelerators and detectors for cancer hadrontherapy and for the production of radioisotopes. Dr. Saverio Braccini Bern, 25.4.2013 i ii Contents Introduction 1 I 5 1 Particle Accelerators and Detectors applied to Medicine 7 2 Particle Accelerators for medical Diagnostics and Therapy 23 2.1 Linacs and Cyclinacs for Hadrontherapy . 23 2.2 The new Bern Cyclotron Laboratory and its Research Beam Line . 39 3 Particle Detectors for medical Applications of Ion Beams 49 3.1 Segmented Ionization Chambers for Beam Monitoring in Hadrontherapy 49 3.2 Proton Radiography with nuclear Emulsion Films . 62 3.3 A Beam Monitor Detector based on doped Silica Fibres . -
Proton Therapy ACKNOWLEDGEMENTS
AMERICAN BRAIN TUMOR ASSOCIATION Proton Therapy ACKNOWLEDGEMENTS ABOUT THE AMERICAN BRAIN TUMOR ASSOCIATION Founded in 1973, the American Brain Tumor Association (ABTA) was the first national nonprofit organization dedicated solely to brain tumor research. For over 40 years, the Chicago-based ABTA has been providing comprehensive resources that support the complex needs of brain tumor patients and caregivers, as well as the critical funding of research in the pursuit of breakthroughs in brain tumor diagnosis, treatment and care. To learn more about the ABTA, visit www.abta.org. We gratefully acknowledge Anita Mahajan, Director of International Development, MD Anderson Proton Therapy Center, director, Pediatric Radiation Oncology, co-section head of Pediatric and CNS Radiation Oncology, The University of Texas MD Anderson Cancer Center; Kevin S. Oh, MD, Department of Radiation Oncology, Massachusetts General Hospital; and Sridhar Nimmagadda, PhD, assistant professor of Radiology, Medicine and Oncology, Johns Hopkins University, for their review of this edition of this publication. This publication is not intended as a substitute for professional medical advice and does not provide advice on treatments or conditions for individual patients. All health and treatment decisions must be made in consultation with your physician(s), utilizing your specific medical information. Inclusion in this publication is not a recommendation of any product, treatment, physician or hospital. COPYRIGHT © 2015 ABTA REPRODUCTION WITHOUT PRIOR WRITTEN PERMISSION IS PROHIBITED AMERICAN BRAIN TUMOR ASSOCIATION Proton Therapy INTRODUCTION Brain tumors are highly variable in their treatment and prognosis. Many are benign and treated conservatively, while others are malignant and require aggressive combinations of surgery, radiation and chemotherapy. -
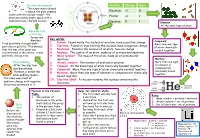
Key Words: 1. Proton: Found Inside the Nucleus of an Atom, Have a Positive Charge 2. Electron: Found in Rings Orbiting the Nucle
Nucleus development Particle Charge Mass This experiment allowed Rutherford to replace the plum pudding Electron -1 0 model with the nuclear model – the Proton +1 1 atom was mainly empty space with a small positively charged nucleus Neutron 0 1 Element All the same type of atom Alpha particle scattering Geiger and Key words: Marsden 1. Proton: Found inside the nucleus of an atom, have a positive charge Compound fired positively-charged alpha More than one type 2. Electron: Found in rings orbiting the nucleus, have a negative charge particles at gold foil. This showed of atom chemically that the mas of an atom was 3. Neutrons: Found in the nucleus of an atom, have no charge bonded together concentrated in the centre, it was 4. Nucleus: The centre of an atom, made up of protons and neutrons positively charged too 5. Mass number: The mass of the atom, made up of protons and neutrons Mixture Plum pudding 6. Atomic number: The number of protons in an atom More than one type After the electron 7. Element: All the same type of atom chemically bonded together of element or was discovered, 8. Compound: More than one type of atom chemically bonded together compound not chemically bound Thomson created the 9. Mixture: More than one type of element or compound not chemically together plum pudding model – bound together the atom was a ball of 10. Electron Shell: A ring surrounding the nucleus containing the positive charge with negative electrons electrons scattered in it Position in the Periodic Rules for electron shells: Table: 1. -

Detection of a Strange Particle
10 extraordinary papers Within days, Watson and Crick had built a identify the full set of codons was completed in forensics, and research into more-futuristic new model of DNA from metal parts. Wilkins by 1966, with Har Gobind Khorana contributing applications, such as DNA-based computing, immediately accepted that it was correct. It the sequences of bases in several codons from is well advanced. was agreed between the two groups that they his experiments with synthetic polynucleotides Paradoxically, Watson and Crick’s iconic would publish three papers simultaneously in (see go.nature.com/2hebk3k). structure has also made it possible to recog- Nature, with the King’s researchers comment- With Fred Sanger and colleagues’ publica- nize the shortcomings of the central dogma, ing on the fit of Watson and Crick’s structure tion16 of an efficient method for sequencing with the discovery of small RNAs that can reg- to the experimental data, and Franklin and DNA in 1977, the way was open for the com- ulate gene expression, and of environmental Gosling publishing Photograph 51 for the plete reading of the genetic information in factors that induce heritable epigenetic first time7,8. any species. The task was completed for the change. No doubt, the concept of the double The Cambridge pair acknowledged in their human genome by 2003, another milestone helix will continue to underpin discoveries in paper that they knew of “the general nature in the history of DNA. biology for decades to come. of the unpublished experimental results and Watson devoted most of the rest of his ideas” of the King’s workers, but it wasn’t until career to education and scientific administra- Georgina Ferry is a science writer based in The Double Helix, Watson’s explosive account tion as head of the Cold Spring Harbor Labo- Oxford, UK. -

Protocol for Heavy Charged-Particle Therapy Beam Dosimetry
AAPM REPORT NO. 16 PROTOCOL FOR HEAVY CHARGED-PARTICLE THERAPY BEAM DOSIMETRY Published by the American Institute of Physics for the American Association of Physicists in Medicine AAPM REPORT NO. 16 PROTOCOL FOR HEAVY CHARGED-PARTICLE THERAPY BEAM DOSIMETRY A REPORT OF TASK GROUP 20 RADIATION THERAPY COMMITTEE AMERICAN ASSOCIATION OF PHYSICISTS IN MEDICINE John T. Lyman, Lawrence Berkeley Laboratory, Chairman Miguel Awschalom, Fermi National Accelerator Laboratory Peter Berardo, Lockheed Software Technology Center, Austin TX Hans Bicchsel, 1211 22nd Avenue E., Capitol Hill, Seattle WA George T. Y. Chen, University of Chicago/Michael Reese Hospital John Dicello, Clarkson University Peter Fessenden, Stanford University Michael Goitein, Massachusetts General Hospital Gabrial Lam, TRlUMF, Vancouver, British Columbia Joseph C. McDonald, Battelle Northwest Laboratories Alfred Ft. Smith, University of Pennsylvania Randall Ten Haken, University of Michigan Hospital Lynn Verhey, Massachusetts General Hospital Sandra Zink, National Cancer Institute April 1986 Published for the American Association of Physicists in Medicine by the American Institute of Physics Further copies of this report may be obtained from Executive Secretary American Association of Physicists in Medicine 335 E. 45 Street New York. NY 10017 Library of Congress Catalog Card Number: 86-71345 International Standard Book Number: 0-88318-500-8 International Standard Serial Number: 0271-7344 Copyright © 1986 by the American Association of Physicists in Medicine All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means (electronic, mechanical, photocopying, recording, or otherwise) without the prior written permission of the publisher. Published by the American Institute of Physics, Inc., 335 East 45 Street, New York, New York 10017 Printed in the United States of America Contents 1 Introduction 1 2 Heavy Charged-Particle Beams 3 2.1 ParticleTypes ......................... -

Present Status of Fast Neutron Therapy Survey of the Clinical Data and of the Clinical Research Programmes
PRESENT STATUS OF FAST NEUTRON THERAPY SURVEY OF THE CLINICAL DATA AND OF THE CLINICAL RESEARCH PROGRAMMES Andre Wambersie and Francoise Richard Universite Catholique de Louvain, Unite de Radiotherapie, Neutron- et CurietheVapie, Cliniques Universitaires St-Luc, 1200-Brussels, Belgium. Abstract The clinical results reported from the different neutron therapy centres, in USA, Europe and Asia, are reviewed. Fast neutrons were proven to be superior to photons for locally extended inoperable salivary gland tumours. The reported overall local control rates are 67 % and 24 % respectively. Paranasal sinuses and some tumours of the head and neck area, especially extended tumours with large fixed lymph nodes, are also indications for neutrons. By contrast, the results obtained for brain tumours were, in general, disappointing. Neutrons were shown to bring a benefit in the treatment of well differentiated slowly growing soft tissue sarcomas. The reported overall local control rates are 53 % and 38 % after neutron and photon irradiation respectively. Better results, after neutron irradiation, were also reported for bone- and chondrosarcomas. The reported local control rates are 54 % for osteosarcomas and 49 % for chondrosarcomas after neutron irradiation; the corresponding values are 21 % and 33 % respectively after photon irradiation. For locally extended prostatic adenocarcinoma, the superiority of mixed schedule (neutrons + photons) was demonstrated by a RTOG randomized trial (local control rates 77% for mixed schedule compared to 31 % for photons). Neutrons were also shown to be useful for palliative treatment of melanomas. Further studies are needed in order to definitively evaluate the benefit of fast neutrons for other localisations such as uterine cervix, bladder, and rectum. -

PIONEERING THERAPY for LIFE Table of Contents
PIONEERING THERAPY FOR LIFE Table of contents Key figures 20151 IBA at a glance 2 IBA is 30 years old 4 Proton therapy 6 Dosimetry 20 RadioPharma Solutions 24 Industrial 26 Human resources 28 Corporate social responsibility 30 Governance 36 Economical review 39 IFRS consolidated financial statements for the year ended December 31, 2015 73 IBA SA Annual financial statements148 General information 152 Stock market and shareholders 157 Key figures 2015 REBIT (3) / SALES & SERVICES TRENDS 12% 12 IBA is a high-technology medical 10.9% company which concentrates 10% 10 its activities on proton therapy, radiopharmacy, particle accelerators 8 8% for industry, and dosimetry. 6% IBA is the worldwide leader in 6 the proton therapy market. 4 4% Listed on the NYSE Euronext Brussels. 2 2% 1 200 employees worldwide. 0% IBA operates in two areas: “Proton 0 Therapy and Other Accelerators ” and 2010 2011 2012 2013 2014 2015 “Dosimetry”. Key figures 2015 + 22.6% 332 2015 revenue increase EUR million Backlog in Proton Therapy & Other Accelerators OPERATING RESULTS 2014 2015 Change CAGR (1) (EUR 000) (EUR 000) (EUR 000) (%) 2014/2015 Sales and services 220 577 270 357 49 780 22.60% Gross margin 96 096 113 655 18.30% REBITDA (2) 28 321 33 710 5 389 19.00% REBITDA/Sales and services 12.80% 12.50% REBIT (3) 22 932 29 553 6 621 28.90% REBIT/Sales and services 10.40% 10.90% Net profit 24 294 61 189 36 895 151.90% (1) CAGR: compound annual growth rate (2) REBITDA: recurring earnings before interest, taxes, depreciation, and amortization. -

8.1 the Neutron-To-Proton Ratio
M. Pettini: Introduction to Cosmology | Lecture 8 PRIMORDIAL NUCLEOSYNTHESIS Our discussion at the end of the previous lecture concentrated on the rela- tivistic components of the Universe, photons and leptons. Baryons (which are non-relativistic) did not figure because they make a trifling contribu- tion to the energy density. However, from t ∼ 1 s a number of nuclear reactions involving baryons took place. The end result of these reactions was to lock up most of the free neutrons into 4He nuclei and to create trace amounts of D, 3He, 7Li and 7Be. This is Primordial Nucleosynthesis, also referred to as Big Bang Nucleosynthesis (BBN). 8.1 The Neutron-to-Proton Ratio Before neutrino decoupling at around 1 MeV, neutrons and protons are kept in mutual thermal equilibrium through charged-current weak interactions: + n + e ! p +ν ¯e − p + e ! n + νe (8.1) − n ! p + e +ν ¯e While equilibrium persists, the relative number densities of neutrons and protons are given by a Boltzmann factor based on their mass difference: n ∆m c2 1:5 = exp − = exp − (8.2) p eq kT T10 where 10 ∆m = (mn − mp) = 1:29 MeV = 1:5 × 10 K (8.3) 10 and T10 is the temperature in units of 10 K. As discussed in Lecture 7.1.2, this equilibrium will be maintained so long as the timescale for the weak interactions is short compared with the timescale of the cosmic expansion (which increases the mean distance between parti- cles). In 7.2.1 we saw that the ratio of the two rates varies approximately as: Γ T 3 ' : (8.4) H 1:6 × 1010 K 1 This steep dependence on temperature can be appreciated as follows.