(Subsigara) Distincta (Fieber, 1848) in the Pyrenees: a First Record for Spain and an Unsolved Taxonomic Puzzle
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
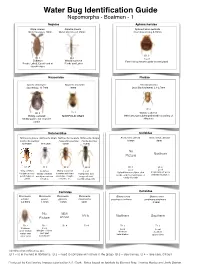
Water Bug ID Guide
Water Bug Identification Guide Nepomorpha - Boatmen - 1 Nepidae Aphelocheridae Nepa cinerea Ranatra linearis Aphelocheirus aestivalis Water Scorpion, 20mm Water Stick Insect, 35mm River Saucer bug, 8-10mm ID 1 ID 1 ID 1 Local Common Widely scattered Fast flowing streams under stones/gravel Ponds, Lakes, Canals and at Ponds and Lakes stream edges Naucoridae Pleidae Ilycoris cimicoides Naucoris maculata Plea minutissima Saucer bug, 13.5mm 10mm Least Backswimmer, 2.1-2.7mm ID 1 ID 1 ID 2 Widely scattered Widely scattered NORFOLK ONLY Often amongst submerged weed in a variety of Muddy ponds and stagnant stillwaters canals Notonectidae Corixidae Notonecta glauca Notonecta viridis Notonecta maculata Notonecta obliqua Arctocorisa gemari Arctocorisa carinata Common Backswimmer Peppered Backswimmer Pied Backswimmer 8.8mm 9mm 14-16mm 13-15mm 15mm 15mm No Northern Picture ID 2 ID 2 ID 2 ID 2 ID 3 ID 3 Local Local Very common Common Widely scattered Local Upland limestone lakes, dew In upland peat pools Ubiquitous in all Variety of waters In waters with hard Peat ponds, acid ponds, acid moorland lakes, or with little vegetation ponds, lakes or usually more base substrates, troughs, bog pools and sandy silt ponds canals rich sites concrete, etc. recently clay ponds Corixidae Corixidae Micronecta Micronecta Micronecta Micronecta Glaenocorisa Glaenocorisa scholtzi poweri griseola minutissima propinqua cavifrons propinqua propinqua 2-2.5mm 1.8mm 1.8mm 2mm 8.3mm No NEW RARE Northern Southern Picture ARIVAL ID 2 ID 2 ID 4 ID 4 ID 3 ID 3 Local Common Local Local Margins of rivers open shallow Northern Southern and quiet waters over upland lakes upland lakes silt or sand backwaters Identification difficulties are: ID 1 = id in the field in Northants. -

Aerial Trapping of Kleidocerys Trunculatus (Or K
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Greenwich Academic Literature Archive Greenwich Academic Literature Archive (GALA) – the University of Greenwich open access repository http://gala.gre.ac.uk __________________________________________________________________________________________ Citation for published version: Reynolds, Don R., Nau, Bernard S. and Chapman, Jason W. (2013) High-altitude migration of Heteroptera in Britain. European Journal of Entomology, 110 (3). pp. 483-492. ISSN 1210-5759 (Print), 1802-8829 (Online) (doi:10.14411/eje.2013.064) Publisher’s version available at: http://dx.doi.org/10.14411/eje.2013.064 __________________________________________________________________________________________ Please note that where the full text version provided on GALA is not the final published version, the version made available will be the most up-to-date full-text (post-print) version as provided by the author(s). Where possible, or if citing, it is recommended that the publisher’s (definitive) version be consulted to ensure any subsequent changes to the text are noted. Citation for this version held on GALA: Reynolds, Don R., Nau, Bernard S. and Chapman, Jason W. (2013) High-altitude migration of Heteroptera in Britain. London: Greenwich Academic Literature Archive. Available at: http://gala.gre.ac.uk/12196/ __________________________________________________________________________________________ Contact: [email protected] This is the Author's Accepted Manuscript version, uploaded in accordance with the publisher's self-archiving policy. Please note: this is the author’s version of a work that was accepted for publication in the EUROPEAN JOURNAL OF ENTOMOLOGY and published 11 July 2013 by the Institute of Entomology (Czech Republic). Changes resulting from the publishing process, such as editing, structural formatting, and other quality control mechanisms may not be reflected in this document. -

Marine Insects
UC San Diego Scripps Institution of Oceanography Technical Report Title Marine Insects Permalink https://escholarship.org/uc/item/1pm1485b Author Cheng, Lanna Publication Date 1976 eScholarship.org Powered by the California Digital Library University of California Marine Insects Edited by LannaCheng Scripps Institution of Oceanography, University of California, La Jolla, Calif. 92093, U.S.A. NORTH-HOLLANDPUBLISHINGCOMPANAY, AMSTERDAM- OXFORD AMERICANELSEVIERPUBLISHINGCOMPANY , NEWYORK © North-Holland Publishing Company - 1976 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise,without the prior permission of the copyright owner. North-Holland ISBN: 0 7204 0581 5 American Elsevier ISBN: 0444 11213 8 PUBLISHERS: NORTH-HOLLAND PUBLISHING COMPANY - AMSTERDAM NORTH-HOLLAND PUBLISHING COMPANY LTD. - OXFORD SOLEDISTRIBUTORSFORTHEU.S.A.ANDCANADA: AMERICAN ELSEVIER PUBLISHING COMPANY, INC . 52 VANDERBILT AVENUE, NEW YORK, N.Y. 10017 Library of Congress Cataloging in Publication Data Main entry under title: Marine insects. Includes indexes. 1. Insects, Marine. I. Cheng, Lanna. QL463.M25 595.700902 76-17123 ISBN 0-444-11213-8 Preface In a book of this kind, it would be difficult to achieve a uniform treatment for each of the groups of insects discussed. The contents of each chapter generally reflect the special interests of the contributors. Some have presented a detailed taxonomic review of the families concerned; some have referred the readers to standard taxonomic works, in view of the breadth and complexity of the subject concerned, and have concentrated on ecological or physiological aspects; others have chosen to review insects of a specific set of habitats. -

I Ventral/Black 28/08/93 Fore I Left ! Ventral! Black 29/08/93 Fore I Right I Ventral I Red
RADAR Research Archive and Digital Asset Repository Geographical and temporal variation of biochemical and colour-pattern polymorphisms in the European moth, Noctua pronuba (L.) Rob Hammond (1994) https://radar.brookes.ac.uk/radar/items/12feb164-d4fa-49d0-b7f9-9d84aa604634/1/ Note if anything has been removed from thesis: Copyright © and Moral Rights for this thesis are retained by the author and/or other copyright owners. A copy can be downloaded for personal non-commercial research or study, without prior permission or charge. This thesis cannot be reproduced or quoted extensively from without first obtaining permission in writing from the copyright holder(s). The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the copyright holders. When referring to this work, the full bibliographic details must be given as follows: Hammond, R (1994), Geographical and temporal variation of biochemical and colour-pattern polymorphisms in the European moth, Noctua pronuba (L.), PhD, Oxford Brookes University WWW.BROOKES.AC.UK/GO/RADAR Geographical and temporal variation of biochemical and colour-pattern polymorphisms in the European moth, Noctua pronuba (L.). Rob Hammond A thesis submitted in partial fulfilment of the requirements of Oxford Brookes University for the degree of Doctor of Philosophy December 1994 Contents: Abstract: .......................................................................................... IV Acknowledgements: ...........................................................................v -

A Two Year Study of Water Bugs at Priory Water
LEICESTERSHIRE ENTOMOLOGICAL SOCIETY A Two-year Study of the Water Bugs (Hemiptera: Heteroptera) of Priory Water NR, Leicestershire Tony Cook 1 & Frank Clark 2 LESOPS 27 (2011) ISSN 0957 - 1019 1Barnwood Cottage, Main Street, Slawston, Market Harborough LE16 7UF (tony.cook20@btinternet,com) 2Bank Cottage, 4 Main Street, Houghton on the Hill LE7 9GD ([email protected]) 1 Introduction Water bugs have been the subject of numerous ecological studies, many of which have focused on the distribution of species in relation to particular features of aquatic habitats. Macan (1954) suggests that the Corixidae is an attractive family for ecological studies because most of the species (nearly 40 in the UK) are common and widely distributed but have restricted habitats. The abiotic and biotic habitat characteristics that influence water bug distributions are complex, involving water chemistry, competition and predation among others. Macan (1938) examined the relationship between corixids and the amount of organic material present in the sediment of lakes and ponds in the Lake District and showed some species occurred only in oligotrophic habitats while others were confined to later successional, eutrophic habitats with higher levels of organic material. Looking at this association from a different point of view, Popham (1943) suggested that the background colour of different habitats influenced distribution with lighter coloured species in low organic matter habitats and vice versa . Savage (1982, 1990) investigated the correlation between corixid distribution and the conductivity of water bodies, conductivity being a measure of the concentration of dissolved ions. This varies widely in freshwater habitats along the oligotrophic– eutrophic spectrum, from below 50µS (micro-Siemens) to about 1,000µS. -

High-Altitude Migration of Heteroptera in Britain
Eur. J. Entomol. 110(3): 483–492, 2013 http://www.eje.cz/pdfs/110/3/483 ISSN 1210-5759 (print), 1802-8829 (online) High-altitude migration of Heteroptera in Britain 1, 2 3 2, 4 DON R. REYNOLDS , BERNARD S. NAU and JASON W. CHAPMAN 1 Natural Resources Institute, University of Greenwich, Chatham, Kent ME4 4TB, UK; e-mail: [email protected] 2 Rothamsted Research, Harpenden, Hertfordshire AL5 2JQ, UK 315 Park Hill, Toddington, Bedfordshire LU5 6AW, UK 4 Environment and Sustainability Institute, University of Exeter, Penryn, Cornwall TR10 9EZ, UK Key words. Heteropteran bugs, aerial sampling, windborne migration, atmospheric transport, life-history strategies, seasonal cycles Abstract. Heteroptera caught during day and night sampling at a height of 200 m above ground at Cardington, Bedfordshire, UK, during eight summers (1999, 2000, and 2002–2007) were compared to high-altitude catches made over the UK and North Sea from the 1930s to the 1950s. The height of these captures indicates that individuals were engaged in windborne migration over distances of at least several kilometres and probably tens of kilometres. This conclusion is generally supported by what is known of the spe- cies’ ecologies, which reflect the view that the level of dispersiveness is associated with the exploitation of temporary habitats or resources. The seasonal timing of the heteropteran migrations is interpreted in terms of the breeding/overwintering cycles of the spe- cies concerned. INTRODUCTION of ~200–300 km in eastern Australia (McDonald & Far- Migratory propensity is highly variable in the Hetero- row, 1988); and the huge numbers of Cyrtorhinus ptera: wing polymorphisms or polyphenisms are common lividipennis (Miridae) and Microvelia spp. -

The Diversity of Feeding Habits Recorded for Water Boatmen
EUROPEAN JOURNAL OF ENTOMOLOGYENTOMOLOGY ISSN (online): 1802-8829 Eur. J. Entomol. 114: 147–159, 2017 http://www.eje.cz doi: 10.14411/eje.2017.020 REVIEW The diversity of feeding habits recorded for water boatmen (Heteroptera: Corixoidea) world-wide with implications for evaluating information on the diet of aquatic insects CHRISTIAN W. HÄDICKE 1, *, DÁVID RÉDEI 2, 3 and PETR KMENT 4 1 University of Rostock, Institute of Biosciences, Chair of Zoology, Universitätsplatz 2, 18055 Rostock, Germany 2 Institute of Entomology, College of Life Sciences, Nankai University, Weijin Rd. 94, 300071 Tianjin, China; e-mail: [email protected] 3 Hungarian Natural History Museum, Department of Zoology, 1088 Budapest, Baross u. 13, Hungary 4 National Museum, Department of Entomology, Cirkusová 1740, CZ-193 00 Praha 9 – Horní Počernice, Czech Republic; e-mail: [email protected] Key words. Heteroptera, Corixoidea, aquatic invertebrates, food webs, diet, predation, feeding habits Abstract. Food webs are of crucial importance for understanding any ecosystem. The accuracy of food web and ecosystem models rests on the reliability of the information on the feeding habits of the species involved. Water boatmen (Corixoidea) is the most diverse superfamily of water bugs (Heteroptera: Nepomorpha), frequently the most abundant group of insects in a variety of freshwater habitats worldwide. In spite of their high biomass, the importance of water boatmen in aquatic ecosystems is fre- quently underestimated. The diet and feeding habits of Corixoidea are unclear as published data are frequently contradictory. We summarise information on the feeding habits of this taxon, which exemplify the diffi culties in evaluating published data on feeding habits in an invertebrate taxon. -

Checklist of Water Bugs (Hemiptera: Heteroptera: Nepomorpha, Gerromorpha) of Slovakia
Eawag_09143 Zootaxa 4058 (2): 227–243 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2015 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.4058.2.5 http://zoobank.org/urn:lsid:zoobank.org:pub:12D13B0A-C2E6-42F0-87DF-A2A041DB8DB6 Checklist of water bugs (Hemiptera: Heteroptera: Nepomorpha, Gerromorpha) of Slovakia BARBORA REDUCIENDO KLEMENTOVÁ1, PETR KMENT2 & MAREK SVITOK1,3,4 1Department of Biology and General Ecology, Faculty of Ecology and Environmental Sciences, Technical University in Zvolen, T. G. Masaryka 24, 96053 Zvolen, Slovakia. E mail: [email protected] 2Department of Entomology, National Museum, Cirkusová 1740, 19300 Praha 9 Horní Počernice, Czech Republic. E mail: [email protected] 3Eawag, Swiss Federal Institute of Aquatic Science and Technology, Department of Aquatic Ecology, Centre of Ecology, Evolution and Biogeochemistry, Seestrasse 79, 6047 Kastanienbaum, Switzerland. E mail: [email protected] 4 Corresponding author Abstract The water bugs represent a significant component of the freshwater biota, play an important role in trophic webs, and may have considerable economic importance. Nevertheless, systematic research of this group has been underdeveloped in Slo- vakia (central Europe) for decades. This work presents a list of water bug species of Slovakia based on an exhaustive re- view of the literature (time span: 1808–2013) and on more than 14,000 individuals collected during extensive field campaigns (2010–2014) or obtained from insect collections. Fifty-six species belonging to 11 families of Heteroptera were recorded from a total of 767 sites. Seven species were recorded for the first time from Slovakia during our research. -
Notes on the Hemiptera, Coleoptera, Diptera and Other Invertebrates of the Burren, Co
Notes on the Hemiptera, Coleoptera, Diptera and Other Invertebrates of the Burren, Co. Clare and Inishmore, Aran Islands Author(s): I. Lansbury Source: Proceedings of the Royal Irish Academy. Section B: Biological, Geological, and Chemical Science, Vol. 64 (1964 - 1966), pp. 89-115 Published by: Royal Irish Academy Stable URL: http://www.jstor.org/stable/20494883 . Accessed: 08/08/2013 20:45 Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at . http://www.jstor.org/page/info/about/policies/terms.jsp . JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Royal Irish Academy is collaborating with JSTOR to digitize, preserve and extend access to Proceedings of the Royal Irish Academy. Section B: Biological, Geological, and Chemical Science. http://www.jstor.org This content downloaded from 140.203.12.206 on Thu, 8 Aug 2013 20:45:21 PM All use subject to JSTOR Terms and Conditions L 89 ] 7. NOTES ON THE HEMIPTERA, COLEOPTERA, DIPTERA AND OTHER TNVERTEBRATES OF THE BURREN, CO. CLARE AND INISHMORE, ARAN ISLANDS. BY I. LANSBURY Hope Department of Entomology, University Museum, Oxford (Communicated by A. Farrington, M.R.I.A.) [Received 9 MARCH Read 8 JUNE, 1964 Published 22 MARcH, 1965] ABSTRACT Habitats are described and the species of aquatic and semi-aquatic Heteroptera recorded from them are discussed. -
Gerromorpha, Nepomorpha) of Germany Based on DNA Barcodes
From water striders to water bugs: the molecular diversity of aquatic Heteroptera (Gerromorpha, Nepomorpha) of Germany based on DNA barcodes Nadine Havemann1,2, Martin M. Gossner3, Lars Hendrich4, Je`roˆme Morinie`re5, Rolf Niedringhaus6, Peter Scha¨fer7 and Michael J. Raupach1,2 1 Fakulta¨t V, Institut fu¨r Biologie und Umweltwissenschaften (IBU), Carl von Ossietzky Universita¨t Oldenburg, Oldenburg, Lower Saxony, Germany 2 German Centre of Marine Biodiversity, Senckenberg Nature Research Society, Wilhelmshaven, Lower Saxony, Germany 3 Forest Entomology, Swiss Federal Institute for Forest, Snow and Landscape Research, Birmensdorf, Switzerland 4 Sektion Insecta varia, SNSB-Bavarian State Collection of Zoology, Munich, Bavaria, Germany 5 Taxonomic coordinator—German Barcode of Life (GBOL), SNSB-Bavarian State Collection of Zoology, Munich, Bavaria, Germany 6 Department of Biology, Earth and Environmental Sciences, Carl von Ossietzky Universita¨t Oldenburg, Oldenburg, Lower Saxony, Germany 7 B.U.G.S. (Biologische Umwelt-Gutachten Scha¨fer), Telgte, North-Rhine Westphalia, Germany ABSTRACT With about 5,000 species worldwide, the Heteroptera or true bugs are the most diverse taxon among the hemimetabolous insects in aquatic and semi-aquatic ecosystems. Species may be found in almost every freshwater environment and have very specific habitat requirements, making them excellent bioindicator organisms for water quality. However, a correct determination by morphology is challenging in many species groups due to high morphological variability and polymorphisms within, but low variability between species. Furthermore, it is very difficult or even Submitted 7 December 2017 impossible to identify the immature life stages or females of some species, e.g., of the Accepted 14 March 2018 corixid genus Sigara. -

Native and Invasive Freshwater Decapods in the UK: Conservation and Impacts
Native and Invasive Freshwater Decapods in the UK: Conservation and Impacts Paula Joy Rosewarne Submitted in accordance with the requirements for the degree of Doctor of Philosophy The University of Leeds School of Biology September 2013 ii The candidate confirms that the work submitted is her own, except where work which has formed part of jointly authored publications has been included. The contribution of the candidate and the other authors to this work has been explicitly indicated below. The candidate confirms that appropriate credit has been given within the thesis where reference has been made to the work of others. This copy has been supplied on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement © 2013 The University of Leeds and Paula Rosewarne iii Chapter Four is based on a jointly authored publication: Rosewarne, P.J., Mortimer, R.J.G. & Dunn, A. M. (2013) Size-dependent impacts of the endangered white-clawed crayfish (Austropotamobius pallipes) (Lereboullet) on the littoral community, Knowledge and Management of Aquatic Ecosystems, 409, 06, p.1-10 P. Rosewarne formulated the idea, conducted the experiment, analysed the data and wrote the manuscript. A. Dunn and R. Mortimer supervised the research and contributed to writing the manuscript. Chapter Five is based on a jointly authored publication: Rosewarne, P., Mortimer, R. & Dunn, A. (2012) Branchiobdellidan infestation on endangered white-clawed crayfish (Austropotamobius pallipes) in the UK. Parasitology, 139, p.774-780. P. Rosewarne collected the data, analysed the data and co-wrote the manuscript. A. Dunn formulated the idea, provided research supervision, advised on data analysis and co-wrote the manuscript. -

Hemiptera: Heteroptera: Nepomorpha, Gerromorpha) of Slovakia
Zootaxa 4058 (2): 227–243 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2015 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.4058.2.5 http://zoobank.org/urn:lsid:zoobank.org:pub:12D13B0A-C2E6-42F0-87DF-A2A041DB8DB6 Checklist of water bugs (Hemiptera: Heteroptera: Nepomorpha, Gerromorpha) of Slovakia BARBORA REDUCIENDO KLEMENTOVÁ1, PETR KMENT2 & MAREK SVITOK1,3,4 1Department of Biology and General Ecology, Faculty of Ecology and Environmental Sciences, Technical University in Zvolen, T. G. Masaryka 24, 96053 Zvolen, Slovakia. E-mail: [email protected] 2Department of Entomology, National Museum, Cirkusová 1740, 19300 Praha 9 – Horní Počernice, Czech Republic. E-mail: [email protected] 3Eawag, Swiss Federal Institute of Aquatic Science and Technology, Department of Aquatic Ecology, Centre of Ecology, Evolution and Biogeochemistry, Seestrasse 79, 6047 Kastanienbaum, Switzerland. E-mail: [email protected] 4 Corresponding author Abstract The water bugs represent a significant component of the freshwater biota, play an important role in trophic webs, and may have considerable economic importance. Nevertheless, systematic research of this group has been underdeveloped in Slo- vakia (central Europe) for decades. This work presents a list of water bug species of Slovakia based on an exhaustive re- view of the literature (time span: 1808–2013) and on more than 14,000 individuals collected during extensive field campaigns (2010–2014) or obtained from insect collections. Fifty-six species belonging to 11 families of Heteroptera were recorded from a total of 767 sites. Seven species were recorded for the first time from Slovakia during our research.