Abstract Book
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Report on Two Day International Webinar on Impact of Covid-19
Report on ‘Two Day International Webinar on ‘Impact of Covid-19 Pandemic on Global Economy’ 22-23 June, 2020 Organised by Centre for Development Studies Department of Economics Rajiv Gandhi University, Arunachal Pradesh Part – I Organising Committee Chief Patron Prof. Saket Kushwaha, Vice-Chancellor, Rajiv Gandhi University Patrons Prof. Amitava Mitra, Pro Vice-Chancellor, Rajiv Gandhi University Prof. Tomo Riba, Registrar, Rajiv Gandhi University Advisors Prof. Tana Showren, Dean, Faculty of Social Sciences Prof. N.C. Roy, Professor, Department of Economics Prof. S.K. Nayak, Professor, Department of Economics Organising Chairperson/Convener Prof. Vandana Upadhyay, Head, Professor, Department of Economics Coordinator Dr. Maila Lama, Sr. Assistant Professor, Department of Economics Deputy Coordinator Dr. Dil. B. Gurung Assistant Professor, Department of Economics Assistant Coordinators Dr. Lijum Nochi, Sr. Assistant Professor, Department of Economics Dr. Anup Kr. Das, Sr. Assistant Professor, Department of Economics Dr. Prasenjit B. Baruah, Sr. Assistant Professor, Department of Economics 1 Part – II Seminar/ Workshop / Webinar / FDP /STPs etc. 2.1: Background / Concept Notes and Objectives The world has been affected by the novel coronavirus (Covid-19) pandemic since November 2019. The virus causes respiratory diseases in human beings from common cold to more rare and serious diseases such as the Severe Acute Respiratory Syndrome (SARS) and the Middle East Respiratory Syndrome (MERS), both of which have high mortality rates (WHO 2020). The UN Secretary General described it as the worst crisis being faced by mankind since World War-II. It may lead to enhanced instability, unrest and enhanced conflict (The Economic Times, April 1, 2020). There is a high risk associated with this disease as it is highly fatal and contagious. -

Selected Candidates
Revised Notification Date: 09/02/2021 Selected Candidates Online Interdisciplinary Refresher Course on Curriculum and Pedagogy Organized by Teaching Learning Centre (Under PMMMNMTT Scheme, Ministry of Education) Tezpur University (February 15- March 1, 2021) Last date for payment of fee extended till 14th February 2021 The following candidates are selected based on information collected from their google form submissions and NMTT portal. It is assumed that all applicants are in active service in HEI. This is to be noted that the programme is aimed only for the persons engaged in HEIs. That is why Students, Research Scholars, School Level teachers etc., are not selected. All selected candidates need to pay a registration fee of Rs. 700/- .The details for payment and upload of necessary information using google form are given below. Please note that the uploaded payment transaction receipt in the form should show at least last four digits of the credited account. Successful completion of the payment procedure and submission of the details thereafter is mandatory. The program will be managed through Google Classroom. The candidates will receive detailed instructions to join Google Classroom via their individual emails shortly after submission of payment details. # Name of the Applicant Designation Name of the Institution 1 BILAL AHMAD KHAN PROGRAMME MANAGER NATIONAL CHILD LABOUR PROJECT SRINAGAR 2 DR. RINTI DUTTA Assistant Professor Lakhimpur Commerce College 3 DR. NITASHREE BARMAN Assistant Professor Pandit Deendayal Upadhyaya Adarsha Mahavidyalaya, Tulungia 4 ATANU KUMAR MISHRA Assistant Professor Pandit Deendayal Upadhyaya Adarsha Mahavidyalaya, Tulungia 5 HARIPRIYA DUTTA Assistant Professor Pandit deendayal Upadhyaya Adarsha Mahavidyalaya Tulungia 6 DR.IMRUL HUSSAIN Assistant Professor Nabajyoti College,Kalgachia 7 DEVAJANI BAKALIAL Assistant Professor Gargaon College 8 PALASH NATH Assistant Professor Rukasen College Bakalia 9 DR. -

Eligibility Circular No 205 of 2021 30.08.2021.Pdf
SAVITRIBAI PHULE PUNE UNIVERSITY (Formerly University of Pune) CIRCULAR No. 205 of 2021 INFORMATION REGARDING ELIGIBILITY CRITERIA FOR VARIOUS UNIVERSITY COURSES. Website : http://unipune.ac.in Dr. Nitin Karmalkar Hon’ble Vice-Chancellor, Savitribai Phule Pune University Dr. N. S. Umarani Hon’ble Pro-Vice-Chancellor, Savitribai Phule Pune University Eligibility Staff Members 1. Smt. M. S. Uttekar Asstt. Registrar Ph. : 25621180 2. Smt M. J. D’souza Asstt. Section Officer Ph. : 25621181 3. Smt. G. J. Zagade Assistant Ph. : 25621183 4. Smt. B. T. Patil Assistant Ph. : 25621182 5. Shri. B. C. Gajalwar Assistant Ph. : 25621187 INDEX Sr. No. Title Page No. 1.Circular .... 1-7 2. Course wise and yearwise No of Students Enrolled .... 8 3. Eligibility Equaivlence Letter & Government Resolution .... 9 - 19 for equivalence of I.T.I. 4. Faculty of Science and Technology 1. Science .... 20-38 2. Engineering .... 39-62 3. Technology .... 63-65 4. Architecture .... 66-67 5. Pharmaceutical Sciences .... 68-69 5. Faculty of Commerce and Management 1. Commerce .... 70-74 2. Management .... 75-78 6. Faculty of Humanities 1. Arts .... 79 - 83 2. Mental Moral & Social Sciences .... 84-89 3. Law .... 90 - 94 7. Faculty of Interdisciplinary Studies 1. Education .... 95 - 98 2. Physical Education .... 99 - 102 3. Library Science .... 103 4. Fine & Performing Arts .... 104 5. Social Work & Journalism .... 105 8. M.Phil. & Ph.D. .... 106-107 9. Definition .... 108-109 10. Instructions For the Final List of Eligibility Chart I .... 110-111 11. Annexure ‘A’ Eligibility Fee .... 112 12. Procedure for Migration Certificate Application .... 113 13. List of U.G.C Recognized University ... -
Intellectual Property As a Strategic Tool for Regional Development
National Webinar On Intellectual Property as a Strategic Tool for Regional Development Organized by Department of Political Science & IQAC Dhakuakhana College In Collaboration With DPIIT IPR Chair, Intellectual Property Right Cell, Tezpur University Date: 18 June, 2021 ‘I have an Innovation... How do I Protect it in the market???’, on this note, Department of Political Science & IQAC Dhakuakhana College, Dhakuakhana in collaboration with the Tezpur University Intellectual Property Rights Cell, Tezpur, organized a National Level Webinar on a very important and relevant issue, ‘Intellectual Property As A Strategic Tool For Regional Development’. The Webinar started with a Inaugural Speech by the Chairperson of the organizing committee and Principal of Dhakuakhana College, Dr. Jugananda Sut, who gave importance on promotion, creation, protection & enforcement of the Intellectual Property Rights amongst various stakeholders. Dr. Sut also focused on establishing a holistic atmosphere, conducive to exploiting the full potential of IP for social, economic and cultural development, and to strengthen IP Chairs in educational institutions of higher learning to provide quality teaching and research, develop teaching capacity and curriculum and evaluate the work on performance-based criteria. ‘Why is IP important to us?’ on this note the Key Note Speaker of the Webinar, Prof. Pritam Deb, DPIIT Chair Professor, IPR Cell, Tezpur University, highlighted how in today’s globally competitive environment, Intellectual Property (IP) has placed itself on a pedestal in the context of economic growth and is becoming increasingly important. Prof. Deb also tinted how the increasing significance of intangible assets in the global economy is forcing business organizations to actively manage their IPR as a key driver for building and sustaining their competitive advantage and achieving superior performance and development. -
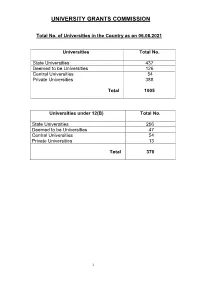
Consolidated List of All Universities
UNIVERSITY GRANTS COMMISSION Total No. of Universities in the Country as on 06.08.2021 Universities Total No. State Universities 437 Deemed to be Universities 126 Central Universities 54 Private Universities 388 Total 1005 Universities under 12(B) Total No. State Universities 256 Deemed to be Universities 47 Central Universities 54 Private Universities 13 Total 370 1 S.No ANDHRA PRADESH Date/Year of Notification/ Establishment 1. Acharya N.G. Ranga Agricultural University, Lam, 1964 Guntur – 522 034,Andhra Pradesh (State University) 2. Acharya Nagarjuna University, Nagarjuna Nagar-522510, Dt. Guntur, 1976 Andhra Pradesh. (State University) 3. Adikavi Nannaya University, 25-7-9/1, Jayakrishnapuram, 2006 Rajahmundry – 533 105, East Godavari District, Andhra Pradesh. (State University) 4. Andhra University, Waltair, Visakhapatnam-530 003, Andhra Pradesh. 1926 (State University) 5. Bharatiya Engineering Science and Technology Innovation University, 17.02.2019 Gownivaripalli, Gorantla Mandal, Anantapur, Andhra Pradesh (Private University) 6. Central University of Andhra Pradesh, IT Incubation Centre Building, 05.08.2019 JNTU Campus, Chinmaynagar, Anantapuramu, Andhra Pradesh- 515002 (Central University) 7. Central Tribal University of Andhra Pradesh, Kondakarakam, 05.08.2019 Vizianagaram, Andhra Pradesh 535008 (Central University) 8. Centurion University of Technology and Management, Gidijala Junction, 23.05.2017 Anandapuram Mandal, Visakhapatnam – 531173, Andhra Pradesh. (Private University) 9. Damodaram Sanjivayya National Law University, Plot No. 116, Sector 2008 11 MVP Colony, Visakhapatnam – 530 017, Andhra Pradesh. (State University) 10. Dr. Abdul Haq Urdu University, Kurnool- 518001, Andhra Pradesh 14.12.2018 (State University) 11. Dr. B.R. Ambedkar University, Etcherla, Dt. Srikakulam-532410, 2008 Andhra Pradesh. (State University) 12. Dravidian University, Srinivasanam, -517 425, Chittoor District, 1997 Andhra Pradesh. -

Time Schedule
NATIONAL SEMINAR ON SCIENCE FOR SUSTAINABLE DEVELOPMENT (SSD-2020) 25th & 26th September, 2020 Time Schedule Day 1 25th September Inaugural Session 9.30-10.00 Joining the Seminar on Zoom 10.00-10.05 Welcome Address 10.05-10.15 Inaugural Lecture Dr. Arup Kumar Misra Director, Assam Science Technology & Environment Council (ASTEC) 10.15-10.25 Speech by Guest of Honour Prof. Dibakar Chandra Deka Honourable Vice Chancellor, Madhabdev University 10.25-10.55 Key Note Address Prof. Bhupendra Nath Goswami SERB Distinguished Fellow, Department of Physics, Cotton University 10.55-11.00 Vote of thanks Break Session 1 11.25-11.30 Chair: Prof. Dibakar Chandra Deka Honourable Vice Chancellor, Madhabdev University 11.30-12.00 IL1: Prof. Ghanashyam Bez Department of Chemistry, NEHU Shillong 12.00-12.30 IL2: Prof. Ramesh Chandra Deka Department of Chemical Sciences, Tezpur University 12.30-13.00 IL3: Prof. Prodeep Phukan Department of Chemistry, Gauhati University Lunch Break Session 2 14.25-14.30 Chair: Dr. Saroj Kumar Bhattacharyya Mark Wainwright Analytical Centre, University of New South Wales, Australia 14.30-14.45 SIL 1: Dr. Bipul Sarma Department of Chemcal Sciences, Tezpur University 14.45-15.00 SIL 2: Dr. Ankur Kanti Guha Department of Chemistry, Cotton University 15.00-15.10 OP 1: Rinki Moni Kalita Research Scholar, Department of Chemistry, Gauhati University 15.10-15.20 OP 2: Rahul Kumar Sarma Baruah Research Scholar, Department of Applied Sciences, Gauhati University 15.20-15.30 OP 3: Sarojmoni Kalita Research Scholar, Department of Chemistry, Gauhati University 15.30-15.40 OP 4: Dr. -

For Waiver of A'dmission Fees, Tution Fees Etc. for Students Taking Admission in H.S.Ru.G.(B.A.Lb.Sc./B.Com.) and P.G
GOVERNMENT OF ASSAM OFFICE OF TIIE DIRECTOR OF HIGIIER EDUCATION,ASSAM No. PC/HE/lvIis c. I 42 12O20 I 5 | Dated Kahilipara the 6ft September,202l From Sri Dharma Kanta Mili, A.C.S Director of Higher Education, Assam Kahilipara Guwahati- I 9. r'!/ 1. The Registrar, Gauhati University/ Dibrugarh University/ Bodoland University/ Cotton University/ K.B.V. Sanskrit & Ancient Studies University/ Bhattadev University/ Madhabdev University/ Rabindranath Tagore University/ Women University/I,Iajuli University of Culture/ Birangana Sati Sadhini Raijyik Viswavidyalaya. 2.The Principal(s), All Govt. College/Provincialised College/Govt. Model College. of Assam. Sub: Reg. Scheme for waiver of Admission fees, tution fees etc. for students taking admission in H.S.AJ.G.(B.A./B.Sc./B.Com. and P.G. (M.A./lvI.Sc./lvl.Com.) course for the year 2021-22 . Ref. Govt. O.M. No.AHE35412021 18, dtd.06l09 12021. Sir, In inviting a reference to the subject cited above, I have the honour to enclose herewith the Govt. Office Memorandum No. AHE.3541202118, dtd.06/09/2021 regarding Scheme for waiver of A'dmission fees, tution fees etc. for students taking admission in H.S.ru.G.(B.A.lB.Sc./B.Com.) and P.G. (M.A./1vI.Sc./M.Com.) courses for the year 2021-22 for your kind information and necessary action. Yours Faithfirlly, -d's Director of Higher Education, Assam. Ikhilipara Guwahati- 19. Memo No. PCtHElMisc.l42lz020l5l-A Dated Kahilipara the 6th September,202l Copy to :- L The P.S. to the Hon'ble Minister, Education, Assam, Dipur, Guwahati-6 for kind appraisal of the Hon'ble Minister. -

Regarding Uploading of Fee Waiver Datas in Web Portal
REMINDER-I GOVERNMENT OF ASSAM OFFICE OF THE DIRECTOR OF HIGHER EDUCATION,ASSAM KAHILIPARA ***+* GUWAHATI-l 9. No.pC/HE/plan-Z4lZ01ZlZl0 Dated Kahilipara the l2th September /2019 From Smti. G. Phukan, ACS Director of Higher Education, Assam Kahilipara, Guwahati- I 9. To, l. The Registrar, Gauhati University, Guwahati/ Dibrugarh University, Dibrugarh/ Bodoland University, Kokrajhar/ Cotton University, Guwahati/ Kumar Bhaskar Varma Sanskrit & Ancient Studies University, Nalbari/ Bhattadev University, Bajali./Madhabdev University, Narayanpur/ Kabi Guru Rabindra Nath Tagore University, Hojai. ffrn"Principal (all), Govt. and Provincialised Colleges of Assam. Sub Regarding uploading of fee waiver datas in web portal. Ref. Th i s offi ce letter N o. PC I HE lPlan-24 I 20 I 2 I 206, dtd.1 2 I 09 120 1 9 Sir, In continuation to this office letter mentioned under reference, I would like to inform you that the documents/data have not yet been uploaded in the fee waiver web portal developed through AMTRON for the year 2019-20. You are, therefore requested to upload the documents/data in the fee waiver web portal for zllg-zLlatest by 20th Septemberl}Dlg, so that the process can be completed immediately and the undersigned can move proposal to Govt. for release of fund at the earliest. The steps to be followed for uploading fee waiver datas noted below : 1. Login and setting up new pass word. 2. Uploading of University/College category and submit the document proof. 3. Approval of University/College category. 4. Institute will prepare and upload the all students Master Data. 5. lnstitute willprepare and upload the BPL students Master Data. -
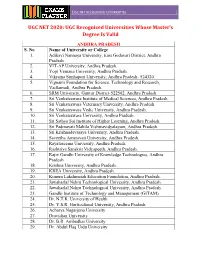
Ugc Net Recognized Universities
UGC NET RECOGNIZED UNIVERSITIES UGC NET 2020: UGC Recognized Universities Whose Master’s Degree Is Valid ANDHRA PRADESH S. No Name of University or College 1. Adikavi Nannaya University, East Godavari District, Andhra Pradesh. 2. VIT-AP University, Andhra Pradesh. 3. Yogi Vemana University, Andhra Pradesh. 4. Vikrama Simhapuri University, Andhra Pradesh- 524320. 5. Vignan's Foundation for Science, Technology and Research, Vadlamudi, Andhra Pradesh. 6. SRM University, Guntur District-522502, Andhra Pradesh. 7. Sri Venkateswara Institute of Medical Sciences, Andhra Pradesh. 8. Sri Venkateswara Veterinary University, Andhra Pradesh. 9. Sri Venkateswara Vedic University, Andhra Pradesh. 10. Sri Venkateswara University, Andhra Pradesh. 11. Sri Sathya Sai Institute of Higher Learning, Andhra Pradesh. 12. Sri Padmavati Mahila Vishwavidyalayam, Andhra Pradesh. 13. Sri Krishnadevaraya University, Andhra Pradesh. 14. Saveetha Amaravati University, Andhra Pradesh. 15. Rayalaseema University, Andhra Pradesh. 16. Rashtriya Sanskrit Vidyapeeth, Andhra Pradesh. 17. Rajiv Gandhi University of Knowledge Technologies, Andhra Pradesh 18. Krishna University, Andhra Pradesh. 19. KREA University, Andhra Pradesh. 20. Koneru Lakshmaiah Education Foundation, Andhra Pradesh. 21. Jawaharlal Nehru Technological University, Andhra Pradesh. 22. Jawaharlal Nehru Technological University, Andhra Pradesh. 23. Gandhi Institute of Technology and Management (GITAM) 24. Dr. N.T.R. University of Health 25. Dr. Y.S.R. Horticultural University, Andhra Pradesh. 26. Acharya Nagarjuna University 27. Dravidian University 28. Dr. B.R. Ambedkar University 29. Dr. Abdul Haq Urdu University UGC NET RECOGNIZED UNIVERSITIES 30. Centurion University of Technology and Management 31. Bharatiya Engineering Science and Technology Innovation University, Andhra Pradesh. 32. Damodaram Sanjivayya National Law University 33. Andhra University, Andhra Pradesh. 34. Central University of Andhra Pradesh ARUNACHAL PRADESH S. -

'Nrvl
GOVERNMENT OF ASSAM OFFICEoFTHEDIRECToRoFHIGHEREDUCATION,ASSAM KAHILIPAIL\/'nrvL {< * * * {< GUWAHATI-Ig.vv ' DatedKahilipara the26th August 12019' No. PC/HE lPlan-24120121206 From Smti. G. Phukan, ACS Director of Higher Education, Assam Kahilipara, Guwahati- 1 9. .Ao, 1. The Registrar, Dibrugarh/ Bodoland University' Gauhati University, Guwahati/ Dibrugarh University, Bhaskar Varma Sanskrit & Ancient Kokrajhar/ Cottorii-lniversity, Cuwanlatil Kumar ghattadev Bajali'/Madhabdev university' studies university, Natuuril university, Hojai' Nuru,urp, rl KabiGuru Rabindra Nath Tagore University' 2.ThePrinciPal (all), Govt. and Provincialised Colleges of Assam' waiver datas in web portal Sub: Regarding uploading of fee Sir, would like to state that the web portal for with reference to the subject cited above, I colleges for the academic session 2019-20 fee waiver datas in respect of Universities and uploading the must previous year. The Universities and colleges authorities has been developed through AMTRON like fee waiver datas to minimise the data eITor' have to follow the following steps for uploading submit the document proof' 1. Updating of University/college category and 2. DHE will verify and approved the College category' Master Data. 3. Institute will prepare and upload the all students Master Data. 4. Institute will prepare and upload the BPL students 5. Entry of Fee Master Data( applicable only for Universities). official seal of concerned authority of the 6. Uploading of Undertaking (that must be signed with institution) ,,Compile Process. (the Compile Data option is 7. The final step is Datd' to complete the Fee Waiver Process" Menu)' available in the Fee waiver Application in the "Admission please contact for any clarification at email via prasanta.lahkar:r'.0.amtron'in Yours 1 Director of Higher Ed the August 12019. -

Kahilipara, Guwahati-19
^ COVERNMENTOF ASSAM, OFFICE OF I}IE DIRECTOR OI HIGHER EDUCATION, ASSAM, KAHII-IPARA, GUWAHATI-19. No.Ec/Apptt24l2015/93 oated Kahilipara, rhe 0101-2020 From: - Smti G. phukan. ecs Director of Higher Educarion, Assam Kahilipara, Guwahati-19. T A. The Registrar, 1. Cauhari Universfty, Jalukbarl, Ghy_14, 2. Dibrugalh University, Dibrugarh, 3. Cotton University, pa.nbazar, Ghy_o1, 4. BodolandUniversity,Kokrajhar, 5. Kumar Bhaskarverma Sanskrit & Ancient Studies University, Nalbari, 6. K.K. Handique State Open University, Guwahati. 7. Assam University, Silchar, Cachar. .lbzpur. 8. Tezpur Umversj ty, 9. Assam Women,s University, Jorhat. 10. Mahapurusha Srimanta Sankaradeva Viswavidyalaya, Nagaon. 11. K shnaguru Adhyatmrk Vjswavidyalava 12. Bhatta Devuniversity pathsal4 13. Srl Sri Madhabdev University, Narayan pur Viswavidyalaya, 14. Kabiguru Rabindranath Tegore University, Hojai B, The Principal, All Provmcialsed & Covt. Collges/Mahavidyalaya. Sub :- Awareness regarding Govt. of Assam,s 104 services by piramal Sr,,asthya. Ref :- Covt. letter No. PMSRI I ASIS AR/20L9-20/017 Dared 2Z /1.1 l2}1g Sir,4vIadam, With relerence to the letter on the subiect cited above. I have the honor to forwarci herewith a copy of letter and its relevant document received from Hardeep Singh Bambrah, Head- Assam & Norlheast Piramal Swasthya Management and Research Instifute Bamunimaidam Gauhati 21 m connection with awareness regarding Govt. of Assam's 104 sewices by Piramal Swasthya which is sela-explanatory for taking necessary action from your end. This is lor favour of your kind in{ormation and necessary action. Yours' rhtully a9 I Director of I Kahilipara, Guw 9 Memo No.EG/Apptt/24l2015/93-A Kahilipara, fte 03-01-2020 Copy to :. -
International Conference on RECENT TRENDS in PHARMACEUTICAL SCIENCES & PROSPECTS of ETHNOMEDICINE Scheduled to Be Held on 4Th & 5Th June, 2020
Invitation Dear Sir/Madam, It gives us immense pleasure to inform you that the Department of Medical Laboratory Technology, Nowgong College in association with ICMR-Regional Medical Research Centre, N.E. Region, Dibrugarh, Assam Science Technology & Environment Council, DST, Govt. of Assam & North Eastern Institute of Ayurveda & Homeopathy, Shillong, India is going to organize an International Conference on RECENT TRENDS IN PHARMACEUTICAL SCIENCES & PROSPECTS OF ETHNOMEDICINE scheduled to be held on 4th & 5th June, 2020. Your Kind cooperation and active participation in the conference is cordially solicited. You and your esteemed colleagues’ presence and contribution in form of research articles will bring the conference a grand success. With regards Dr. Sarat Borkataki Principal/President Nowgong College, Nagaon, Assam E-mail: [email protected] Convenor Dr. Krishna Kanta Medhi Dean, PG Classes, Nowgong College E-mail: [email protected] Phone: 7002546286 Organizing Secretary Dr. Bhuban Ch. Chutia Department of Zoology, Nowgong College E-mail: [email protected] Phone: 7002133245 Joint Organizing Secretary Dr. Himangshu Baruah Department of Rasa Shastra & Bhasiajya Kalpana, NEIAH, Shillong E-mail: [email protected] Phone: 8638328913 Secretary (s) Publicity Dr. Farishta Yasmin Department of Botany, Nowgong College Email: [email protected] & Dr. Parikshit Gogoi Department of Chemistry, Nowgong College Email: [email protected] INTERNATIONAL CONFERENCE ORGANIZING COMMITTEE Advisor(s) President, Governing Body, Nowgong College Co-ordinator, IQAC, Nowgong College Prof. P.J. Handique, Vice Chancellor, Gauhati University Prof. Dibakar Ch. Deka, Vice Chancellor, Madhabdev University Dr. N. C. Talukdar, Director, IASST, Guwahati Prof. Veena Tandon, Former Professor, Dept. of Zoology, NEHU & NASI Senior Scientist Prof. S.K.