Phytoremediation Study of Aquatic Macrophytes Collected from Five Lakes of Bangalore, India
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bangaluru.Qxp:Layout 1
BENGALURU WATER SOURCES THE WATER-WASTE PORTRAIT Hesaraghatta reservoir Despite its highrises and malls, the ‘Silicon Valley’ 18 km and ‘Garden City’ of India fares badly as far as Arkavathi river infrastructure is concerned, and has lost its famous lakes to indiscriminate disposal of waste and encroachment Chamaraja Sagar BENGALURU reservoir TG HALLI 35 km WTP Boundary under Bangalore Development Authority V-Valley Boundary under Comprehensive Development Plan TK HALLI Sewage treatment plant (STP) WTP Shiva anicut STP (proposed) Cauvery river 90 km (Phase I - Stage 1-4) (future source: Phase II by 2011-14) Water treatment plant (WTP) Hesaraghatta Sewage pumping stations tank Ganayakanahalli Kere Baalur Kere Waterways Disposal of sewage Yelahanka tank Dakshina Pinakini river Waterbodies YELAHANKA Jakkur tank Doddagubbi Kumudvathi river tank Rampur HEBBAL tank Arkavathi river JAKKUR NAGASANDRA Yelamallappachetty Mattikere Hennur Kere tank SPS RAJA CANAL K R PURAM Sadarmangal Ulsoor tank Chamaraja Sagar tank reservoir Byrasandra tank CUBBON PARK V Valley Vrishabhavathi river KADABEESANAHALLI SPS Vartur tank MYLASANDRA KEMPAMBUDHI LALBAGH Hosakerehalli Bellandur tank K & C VALLEY tank V-VALLEY Arkavathi river MADIVALA Bomanahalli tank Nagarbhavi river Begur Hulimavu tank tank Muttanallur Kere Source: Anon 2011, 71-City Water-Excreta Survey, 2005-06, Centre for Science and Environment, New Delhi 304 KARNATAKA THE CITY Municipal area 561 sq km Total area 740 sq km Bengaluru Population (2005) 6.5 million Population (2011), as projected in 2005-06 7.5 million THE WATER Demand angalored’, a slang for being rendered jobless, is a term Total water demand as per city agency (BWSSB) 1125 MLD (2010) made famous by the city’s outsourcing business; Per capita water demand as per BWSSB 173 LPCD ironically, this very business has brought jobs and Total water demand as per CPHEEO @ 175 LPCD 1138 MLD ‘B Sources and supply growth to the capital city of Karnataka. -

New Vtp Applicants List
Contact Person Date of Name Address City District PinCode Telephone Mobile Email Contact Person Name VTP CP Email Mobile Application RURAL DEVELOPMENT AND TRAINING SRIRANGA nithyananda_mv@yah OPP SBM BANK, MAIN ROAD Mandya 571438 08236-252334 9845446401 [email protected] NITHYANANDA MV 9845446401 15-Apr-15 SOCIETY(R) PATNA oo.in RURAL DEVELOPMENT AND TRAINING SRIRANGA nithyananda_mv@yah OPP SBM BANK, MAIN ROAD Mandya 571438 08236-252334 9845446401 [email protected] NITHYANANDA MV 9845446401 15-Apr-15 SOCIETY(R) PATNA oo.in BENGALU [email protected] RACHANA ENTERPRISES PLOT NO-15, ABOVE CORPORATION BANK, KENGARI Bangalore 560074 080-28437482 9620400770 [email protected] UMA RUDRESH 9972920022 15-Apr-15 RU m # 2934/25 E 2ND FLOOR ABOVE HDFC BANK CLUB ROAD BANGALO [email protected] raghunathv@sriakshay SRI AKSHAY TECHNOLOGIES Bangalore 560040 080-41493098 9739011252 RAGHUNATHA.V 9739011252 15-Apr-15 VIJAYANAGAR RE m tech.com # 2934/25 E 2ND FLOOR ABOVE HDFC BANK CLUB ROAD BANGALO [email protected] raghunathv@sriakshay SRI AKSHAY TECHNOLOGIES Bangalore 560040 080-41493098 9739011252 RAGHUNATHA.V 9739011252 15-Apr-15 VIJAYANAGAR RE m tech.com RURAL DEVELOPMENT AND TRAINING SRIRANGA nithyananda_mv@yah OPP. SBM BANK , MAIN ROAD Mandya 571438 08236-252334 9845446401 [email protected] NITHYANANDA M V 9845446401 15-Apr-15 SOCIETY(R) PATNA oo.in # 2934/25 E 2ND FLOOR ABOVE HDFC BANK CLUB ROAD BANGALO [email protected] raghunathv@sriakshay SRI AKSHAY TECHNOLOGIES Bangalore 560040 080-41493098 9739011252 RAGHUNATHA.V -

Unpaid Dividend-17-18-I3 (PDF)
Note: This sheet is applicable for uploading the particulars related to the unclaimed and unpaid amount pending with company. Make sure that the details are in accordance with the information already provided in e-form IEPF-2 CIN/BCIN L72200KA1999PLC025564 Prefill Company/Bank Name MINDTREE LIMITED Date Of AGM(DD-MON-YYYY) 17-JUL-2018 Sum of unpaid and unclaimed dividend 696104.00 Sum of interest on matured debentures 0.00 Sum of matured deposit 0.00 Sum of interest on matured deposit 0.00 Sum of matured debentures 0.00 Sum of interest on application money due for refund 0.00 Sum of application money due for refund 0.00 Redemption amount of preference shares 0.00 Sales proceed for fractional shares 0.00 Validate Clear Proposed Date of Investor First Investor Middle Investor Last Father/Husband Father/Husband Father/Husband Last DP Id-Client Id- Amount Address Country State District Pin Code Folio Number Investment Type transfer to IEPF Name Name Name First Name Middle Name Name Account Number transferred (DD-MON-YYYY) 49/2 4TH CROSS 5TH BLOCK MIND00000000AZ00 Amount for unclaimed and A ANAND NA KORAMANGALA BANGALORE INDIA Karnataka 560095 54.00 23-May-2025 2539 unpaid dividend KARNATAKA 69 I FLOOR SANJEEVAPPA LAYOUT MIND00000000AZ00 Amount for unclaimed and A ANTONY FELIX NA MEG COLONY JAIBHARATH NAGAR INDIA Karnataka 560033 72.00 23-May-2025 2646 unpaid dividend BANGALORE ROOM NO 6 G 15 M L CAMP 12044700-01567454- Amount for unclaimed and A ARUNCHETTIYAR AKCHETTIYAR INDIA Maharashtra 400019 10.00 23-May-2025 MATUNGA MUMBAI MI00 unpaid -

To.No.ADM-I / 05 /2020 Office of the Chief Metropolitan Magistrate, Bengaluru, Date 26.05.2020
To.No.ADM-I / 05 /2020 Office of the Chief Metropolitan Magistrate, Bengaluru, Date 26.05.2020 SPECIAL NOTIFICATION Sub: Allocation of business to the newly established XXXVI, XXXVII, XXXVIII, XXXIX, XL & XLI ACMM Courts, Bengaluru –reg. Ref:- 1. G.O. Nos. LAW 137 LCE 2014 dated 26.03.2015 & LAW 137 LCE 2014 dated 28.03.2016 creating the Courts of XXXVI, XXXVII, XXXVIII, XXXIX, XL & XLI ACMM Courts, Bengaluru. 2. Posting of officers to the newly established 06 ACMM Courts vide Notification No. GOB (I)/4(2)/2020 dated 30.04.2020 of the Hon'ble High Court of Karnataka, Bengaluru. 3. Letter No. ADM-I(A)/251/2020 dated 15.05.2020 and ADM-I(A)/256/2020 dated 21.05.2020 of the Registrar, City Civil Court, Bengaluru. * * * In Consultation with the Hon'ble Principal City Civil and Sessions Judge, Bengaluru and in exercise of powers conferred under Section 19 (3) of Criminal Procedure Code 1973, I, ROOPA R. KULKARNI , Chief Metropolitan Magistrate, Bengaluru, do hereby issue this Special Notification regarding allocation of business to the newly established XXXVI, XXXVII, XXXVIII, XXXIX, XL & XLI ACMM Courts as hereunder; “The territorial jurisdiction of the Police Stations along with listed cases as per Annexure-C are hereby withdrawn from respective Courts and assigned to the newly established XXXVII, XXXIX & XLI ACMM Courts, as per Annexure-A”. “The territorial jurisdiction of the Police Stations along with listed cases as per Annexure-C are hereby withdrawn from the respective Courts and assigned to the newly established XXXVI, XXXVIII & XL ACMM Courts, as per Annexure-B”. -

Real Insight Q1 2021 V5
RESEARCH REAL INSIGHT RESIDENTIAL January – March 2021 PROPTIGER RESEARCH Dhruv Agarwala FOREWORD CEO–Elara Group Housing.com | PropTiger.com | Makaan.com The year 2020 was marred due to the economic and social The overall improving economic scenario and consumer repercussions of the COVID-19 pandemic and the subsequent sentiments also underpinned the residential demand that has worldwide lockdowns. However, the beginning of 2021 was nearly bounced back to the pre-COVID levels. A host of marked by a silver lining with the roll out of the much-awaited factors such as low home loan rates, stamp duty cuts in few vaccine. The availability of vaccines has only reinforced states, such as Maharashtra and Karnataka, developer's confidence in the growth outlook providing stronger tailwinds schemes and pent-up demand have definitely improved the for the recovery of the global economy. After all, the sentiments in the first quarter of 2021. Moreover, a slew of International Monetary Fund (IMF) has forecasted the world measures such as increasing the safe harbour limit, tax economy to expand by 6 percent in 2021, after a 3.5 percent exemptions for investments in REITs, reduced interest for contraction in the previous year. Countries such as the US, the aordable housing and many more have been announced by UK, Canada, Australia, China, Japan and India, which were in a the central government for FY 2022, to provide impetus to the recession for most of the previous year, are estimated to show real estate sector in India. positive growth in 2021. While the key indicators and availability of vaccines did set a Among these major economies, India is expected to grow the stage for recovery of the real estate sector in the first quarter, fastest clocking a double-digit growth in 2021. -

Covid-19 Vaccine Registration Procedure
58, Palace Road, Abshot Layout, Vasanth Nagar, Bengaluru, Karnataka 560052 INITIATIVE BY INSTITUTIONAL SOCIAL RESPONSIBILITY CELL(ISRC) Email : [email protected] All Information Provided Under This Is Gathered from Various Sources. Kindly Check Its Authenticity. The College Is not Responsible. .ಗರಹಿಸಲಾ岿ದೆﲂದ ಸﲂಇದರ ಅ蒿ಯಲ್ಲಿ ಒದ岿ಸಲಾದ ಎಲಾಿ ಮಾಹಿ邿ಯನ್ನು ವಿವಿಧ ಮೂಲಗಳ ದಯವಿ糍ನು ಅದರ ಸ郍ಾಾಸ郍ಾ郍ೆಯನ್ನು ಪರಿಶೀಲ್ಲಸಿ. ಇದ响ೆೆ 响ಾಲೆೀಜನ ಜವಾ냍ಾಾರಿಯಲಿ. COVID-19 VACCINE REGISTRATION PROCEDURE CoWIN portal adds four-digit security code: Here is how to register for Covid-19 vaccine The CoWin portal has got a new four-digit security code feature, which will make the vaccination slot booking experience safe and secure. The feature has been added to the CoWin portal after various users had complained of their vaccination certificate getting generated despite them not getting a jab. The CoWIN four-digit security code will look to reduce mistakenly generated vaccination certificates and will also help to prevent scammers from misguiding users. The code must be kept safe and not be shared with anyone. On the vaccination day, you will need to produce the code in order to authenticate the entire vaccination process. Here is how you can register for the Covid-19 vaccine after the introduction of the four-digit security code: 1.Visit the CoWIN portal 2. Register your mobile number by entering your contact details 3. Enter the OTP you get via SMS. If you have already registered, just sign in with required credentials. 4. Enter your location; residence state and district. You can also enter the PIN code of the nearest vaccination centre. -

215AD Bus Time Schedule & Line Route
215AD bus time schedule & line map 215AD DLF Home Town - K.R. Market View In Website Mode The 215AD bus line (DLF Home Town - K.R. Market) has 2 routes. For regular weekdays, their operation hours are: (1) Dlf Home Town: 10:15 AM - 8:50 PM (2) K.R. Market: 5:05 AM - 2:05 PM Use the Moovit App to ƒnd the closest 215AD bus station near you and ƒnd out when is the next 215AD bus arriving. Direction: Dlf Home Town 215AD bus Time Schedule 38 stops Dlf Home Town Route Timetable: VIEW LINE SCHEDULE Sunday 10:15 AM - 8:50 PM Monday 10:15 AM - 8:50 PM K.R.Market (Kalasipalya) E Street, Bangalore Tuesday 10:15 AM - 8:50 PM Makkala Koota Wednesday 10:15 AM - 8:50 PM Mahila Seva Samaja Thursday 10:15 AM - 8:50 PM Friday 10:15 AM - 8:50 PM National College Saturday 10:15 AM - 8:50 PM Basavanagudi Police Station Gunasheela Hospital Southend Circle 215AD bus Info Direction: Dlf Home Town Nanda Talkies Stops: 38 Trip Duration: 51 min Yediyuru Line Summary: K.R.Market (Kalasipalya), Makkala Koota, Mahila Seva Samaja, National College, Basavanagudi Police Station, Gunasheela Hospital, Deepak Nursing Home Southend Circle, Nanda Talkies, Yediyuru, Deepak Nursing Home, Banashankari, Sarakki, Jaraganahalli Banashankari Cross, Yelachenahalli, Metro, Konanakunte Cross, Konanakunte, Sowdamini Kalyana Mantapa, Hari Sarakki Nagara Cross, Glass Factory, Avalahalli (Towards Anjanapura), Nandi Garden Kothanur, Anjanapura, Jaraganahalli Cross Kidwai Layout, 10th Block Anjanapura, Lal Bahaddur Shastri Nagara, Kembathalli, Kembhathahalli Cross, Yelachenahalli Sri Adishakthi -

Tumakuru District
Details of Respective area engineers of BESCOM (Row 2 - District name) (Column 10 - Alphabetical order of Areas) District: Tumakuru Sl No Zone Circle Division Sub Division O&M Unit Areas 1 2 3 4 5 6 7 8 9 10 11 12 Superintending Assistant Executive Assistant Engineer / Name Chief Engineer Name Name Executive Engineer Name Name Engineer Engineer Junior Engineer Tubin kere LM 9071266878 HOSAPALYA, Ammanatti kuppe , KOPPA, Shingatihalli Devarayanapalya, Basavan Mathikere Village BEERGANAHALLI, Amruthur BSNL telephone exchange Puttana palya Borappanahally CHAKENAHALLI, CHIKKAMADHUREY Chikkamadure, Kattigehalli Padavagere DoddamadureColony Sri. Veerabhadrachar GOEDGERE , Holagere pura colony Gollaratti, Kadshettyhalli H P Shettihalli, HANUMAPURA, HOSAHALLI HOSKERE , HOSKERE, Thoobinakere, Sri. Govindappa Sri. Syed Sri. B Gurumurthy (Addnl I/c) 94498 JINNAGARA, JINNAGERE, K.H HALLI, K.T.PALYA, KACHONAHALLI, KADHSHETTA HALLI, KANTHNAHALLI, KELARA, KODALLY , Kaggere village 94482 7900 94482 79016 1 CTAZ 9448461466 TUMAKUR KUNIGAL YD1 43532 AMRUTHURU Nataraj JE (I/c) 9449844129 M Hutuliborasandra, Madurepalya, MANGALA, MANTYA, maratipala, MARKONAHALLI, mavnahalli, kirangur RAGIHALLI, Kirangur SANABA setmkrcircle.work@gm eeetumkur@rediffmail. [email protected] aeebescomyediyuru@g SANABAGATTA, Sheshapura, SHETTIHALLI, SINGATEYHALLI, SINGONAHALLI, Thattekere, Chokkanahalli TOREYHALLI, TUBINKERE, ail.com com mail.com VALALGUNDA, YACHNAHALLI, YADIYUR, Valgere pura KENKERE, Kodipalya, Nagasandra(ramanayakana palya) KODAVATHI aldanahalli Lallapura Valagerepura -
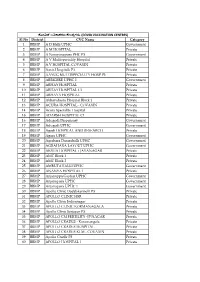
Sl No District CVC Name Category 1 BBMP a D Halli UPHC Government
ಕ ೋ풿蓍 ಲಕಾಕರಣ ಕ ೋᲂ飍ರಗಳು (COVID VACCINATION CENTRES) Sl No District CVC Name Category 1 BBMP A D Halli UPHC Government 2 BBMP A M HOSPITAL Private 3 BBMP A Narayanapura PHC P3 Government 4 BBMP A V Multispeciality Hospital Private 5 BBMP A.V.HOSPITAL COVAXIN Private 6 BBMP Aaxis Hospitals P3 Private 7 BBMP AAYUG MULTISPECIALTY HOSP P3 Private 8 BBMP ABBIGERE UPHC 1 Government 9 BBMP ABHAY HOSPITAL Private 10 BBMP ABHAY HOSPITAL C1 Private 11 BBMP ABHAYA HOSPITAL Private 12 BBMP Abhayahasta Hospital Block 1 Private 13 BBMP ACURA HOSPITAL - COVAXIN Private 14 BBMP Acura Speciality Hospital Private 15 BBMP ADARSH HOSPITAL C1 Private 16 BBMP Adugodi Dispensary Government 17 BBMP Adugodi UPHC Government 18 BBMP Agadi HOSPITAL AND RESEARCH Private 19 BBMP Agara UPHC Government 20 BBMP Agrahara Dasarahalli UPHC Government 21 BBMP AGRAHARA LAYOUT UPHC Government 22 BBMP AKSHA HOSPITAL - JAYANAGAR Private 23 BBMP AMC Block 1 Private 24 BBMP AMC Block 2 Private 25 BBMP AMRUTAHALLI UPHC Government 26 BBMP ANANYA HOSPITAL 1 Private 27 BBMP Anjanappa Garden UPHC Government 28 BBMP Anjanapura UPHC Government 29 BBMP Anjanapura UPHC 1 Government 30 BBMP Apollo Clinic Doddakannelli P3 Private 31 BBMP APOLLO CLINIC HSR Private 32 BBMP Apollo Clinic Indiranagar Private 33 BBMP APOLLO CLINIC KORMANAGALA Private 34 BBMP Apollo Clinic Sarjapur P3 Private 35 BBMP APOLLO CM FERTILITY -JP NAGAR Private 36 BBMP APOLLO CRADLE - Koramangala Private 37 BBMP APOLLO CRADLE HOSPITAL Private 38 BBMP APOLLO CRADLE KLM - COVAXIN Private 39 BBMP Apollo Cradle P3 Private 40 BBMP APOLLO HOSPITAL 1 Private 41 BBMP APOLLO HOSPITAL BG Private 42 BBMP APOLLO HOSPITAL SHESHADRIPURAM Private 43 BBMP APOLLO HOSPITAL-C1 Private 44 BBMP Apollo Hospital-JAYANAGAR Private 45 BBMP APOLLO MEDICAL CENTER P3 Private 46 BBMP APOORVA Block 1 Private 47 BBMP APOORVA Hospital C1 Private 48 BBMP APTS UPHC Government 49 BBMP Arakere Uphc Government 50 BBMP Arka Hospital Private 51 BBMP ARTYEM HOSPITAL Private 52 BBMP ARTYEM HOSPITAL C1 Private 53 BBMP ARYAN MULTISPECIALITY HOS. -

175-Bommanahalli
175-BOMMANAHALLI Part No Name of the BLO Complete Address of the BLO Contact NO. 1 2 3 4 1 C.S.Vinya kumar TI, ARO office,Arakere 9900173812 2 Narasimha Murthy Govt,School,Jaraganahalli 9900000883 3 Somashekar Govt,School,Jaraganahalli 9972995577 4 Dfinesh Kumar Govt,School,Jaraganahalli 9535200096 5 Balaji Govt,School,Jaraganahalli 9980844866 6 Srinivas,N C.S.School Jaraganahalli 9902044096 7 Gangadhar Jyothi School,Jaraganahalli 9945003383 8 Shekhar Govt,School,Jaraganahalli 9448326153 9 Changar Naidu Jyothi School,Jaraganahalli 9343379942 10 Manjunath,N Jyothi School,Jaraganahalli 9880192319 11 Gangadhar Trishul Engkish 6th phese Sarakki 96111510007 12 Sheshadri Manasa School.6th phese Sarakki 9731279223 13 Lakshimi Frank School.6th phese Sarakki 9611046361 14 Nagaraju Frank School.6th phese Sarakki 9945327004 15 Pramila Frank School.6th phese Sarakki 9342499063 16 Mery Trishul Engkish 6th phese Sarakki 9902089738 A.V Education Socity,6th phese 17 Balaji Sarakki 9844444806 A.V Education Socity,6th phese 18 Jhon.M Sarakki 9980798082 19 Rajesh Manasa School.6th phese Sarakki 9886548204 Sadguru Sai baba school, Roopen 20 B.Nagaraju agrahara 21 N.R.Vinod ARO, H.S.R.Layout 9844007186 Sadguru Sai baba school, Roopen 22,23 Jayarajappa agrahara 24,25 N.G.Nataraj BBMP, Bommanahalli Suv division 26,27 Lakkegowda Govt. Primary School, Agara 9945753396 28,29 Manjunatha Govt. Primary School, Agara 9945753396 30,31 P.Jayanti Govt. High School, Agara 9611421222 32,33 Siddappa T.I, ARO, H.S.R. Layout 9449051187 34,35 M.S.Hallur Govt. High School, Agara 9448311640 36 Megha Raj Govt. High School, Agara 9986803706 37 Vinodamma G.H.P.S.Agara 38,39 Padmanabha T.I, ARO, H.S.R. -

V-500CN Bus Time Schedule & Line Route
V-500CN bus time schedule & line map V-500CN Kadugodi Bus Station - Bannerughatta View In Website Mode National Park The V-500CN bus line (Kadugodi Bus Station - Bannerughatta National Park) has 2 routes. For regular weekdays, their operation hours are: (1) Bannerughatta National Park: 6:00 AM - 9:45 PM (2) Kadugodi Bus Station: 8:35 AM - 8:45 PM Use the Moovit App to ƒnd the closest V-500CN bus station near you and ƒnd out when is the next V-500CN bus arriving. Direction: Bannerughatta National Park V-500CN bus Time Schedule 80 stops Bannerughatta National Park Route Timetable: VIEW LINE SCHEDULE Sunday 6:00 AM - 9:45 PM Monday 6:00 AM - 9:45 PM Kadugodi Bus Station Tuesday 6:00 AM - 9:45 PM Kadugodi Bridge SH 35, Bangalore Wednesday 6:00 AM - 9:45 PM Prajwal School Thursday 6:00 AM - 9:45 PM Friday 6:00 AM - 9:45 PM Hope Farm Saturday 6:00 AM - 9:45 PM Bpl Gr Tech Park Itpl I.T.P.L.Whiteƒeld V-500CN bus Info Direction: Bannerughatta National Park Pattandur Agrahara Gate Stops: 80 Trip Duration: 106 min Line Summary: Kadugodi Bus Station, Kadugodi Big Bazaar Bridge, Prajwal School, Hope Farm, Bpl, Gr Tech Park Itpl, I.T.P.L.Whiteƒeld, Pattandur Agrahara Gate, Big I.T.P.L Back Gate Bazaar, I.T.P.L Back Gate, Sathya Sai Hospital, S.J.R Park, Whiteƒeld T.T.M.C. (Vydehi Hospital), Ktpo, I Sathya Sai Hospital Gate Itpl Road, S.A.P.Labs, Graphite India, Kundala Halli Colony, Cmrit College, Aecs Layout Cross, Beml S.J.R Park Gate, Kundalahalli, Kundalahalli Gate, Munnekolalu Cross, Maratahalli Bridge, Marathahalli Bridge Whiteƒeld T.T.M.C. -

NO.HCE.578/2013 High Court of Karnataka BENGALURU DATED: 12 TH MAY 2015
NO.HCE.578/2013 High Court of Karnataka BENGALURU DATED: 12 TH MAY 2015 INTERVIEW FOR GROUP-D POSTS The following applicants who have applied for recruitment to the post of GROUP-D (Peon, Sweepers, Watchmen and Peons (House Keeping)] on the Establishment of High Court of Karnataka, as called for vide Notification No. HCE 578/2013 dated 27.11.2014, are hereby requested to appear for INTERVIEW in the High Court Principal Bench at Bengaluru, High Court Buildings, Bengaluru-560001 on the dates and time mentioned hereunder. Further, you are hereby required to produce all the relevant original certificates regarding qualifications, category (if claimed), proof date of birth, Local Cadre reservation (if claimed) in respect of persons belonging to Hyderabad-Karnataka Region, Proof of identity (i.e., Aadhar Card/Driving License / Election ID etc.,), as mentioned in your call letter alongwith the Character Certificates for verification before Interview. If the same is not produced, the candidature of the applicant is liable to the rejected and they shall not be allowed to take the interview. BY ORDER OF THE HON’BLE THE CHIEF JUSTICE. Sd/- I/c REGISTRAR (RECRUITMENT) TO, The Candidates mentioned in the list annexed hereto Note: The candidates are requested to report at the RECRUITMENT BRANCH near to SBM ATM, of the High Court of Karnataka, Principal bench, Bengaluru . On 25 th May 2015 at 9.00 A.M. 1. DILIP KUMAR R s/o RAMESH D NO.1, NETTIGERE, BOLARE POST, KANAKAPURA MAIN ROAD, BENGALURU SOUTH - 560082., 9980304283 2. PRABHUSHANKAR S s/o SHANKARAPPA H J HOUSING BOARD, HOUSE NO.44, LAKSHMIPURA, ARSIKERE TALUK, HASSAN DIST - 573103., 9036648358 3.