FIREWOOD INSECTS Lee Townsend, Extension Entomologist
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
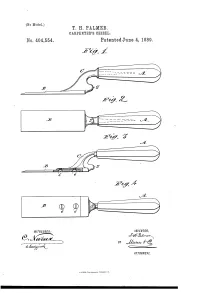
T. H. Palmer. Oarpentbb’S Chisel
(No Model.) T. H. PALMER. OARPENTBB’S CHISEL. _ No. 404,554. I PatentedJune 4, 1889. J , J WITNESSES : 4 ' l/VIg/VTOR ATTOHIVEYJ, UNITED STATES PATENT OFFICE. THE'RON H. PALMER, OF SAN BERNARDINO, CALIFORNIA. CARPENTER’S CHISEL._ SPECIFICATION forming part of Letters Patent No. 404,554, dated June 4, 1889. I Application ?led May 22, 1888.‘ Serial No. 274,724. (No model.) To all whom it may concern; 2, or it may be of a separate piece of mallea Be it known that I, THERON H. PALMER, of ble cast~ir0n or other suitable material and 40 the city and county of San Bernardino, and be joined to the blade B by screws 1) b or oth State of California, have invented a new and erwise. The shank may be secured in the. useful Improvement in Carpenters’ Chisels, handle by a simple tang or in any other de of which the following is a full, clear, and ex sired manner. The trowel-like shape of the act description. ~ > tool provides for cutting gains across a wide 45 This invention consists in a chisel or gouge surface without risk of obstruction by the ' _ for carpenters’ use which has its shank and handle, or the tool may be used as a paring handle portion bent out of line with its blade chisel or for cutting and ?tting in butts or or cutting portion and which is provided with hinges. The crooked shank O is provided at an anvil or hammer-block in rear of the blade its back, as nearly in the same plane as the to form a striking-surface when usingahani cu ttin g-blade as practicable, with aknob-like mer or mallet to force the cutting-tool up to projection S, forming a special anvil or ham its work, instead of striking on the end of mer-block to receive the blows of a hammer the handle direct, which is liable to split or bruise the handle. -

Buying & Storing Firewood & Pellets
Wood Energy Series Fact Sheet FS-937 Buying & Storing Firewood & Pellets 2012 Whether you buy or cut your own firewood, chances are Firewood that you still have plenty left to learn. Even people who have been heating with wood for decades often say “I Firewood dealers come in all shapes and sizes, and wish I knew that years ago!” after reading tips like the although they may appear to be established or ones contained here. questionable, that does not necessarily mean you will be dealt a good or bad hand. Every year, hundreds of thousands of Americans are sold substandard cord wood. This sheet will help you avoid Before You Buy getting a raw deal next time you buy firewood. Find a dealer with a good reputation. Check with friends, the Better Business Bureau, or an online rating site such And if you heat with pellets, there are new developments as www.checkbook.org. Ask for references. you should know that will impact the fuel you buy. Ask the dealer what the moisture content of the wood is Heating Fuel Cost Comparison and how long it has been since it’s been split, not since Prices vary, but you can get an idea of what it will cost it’s been felled. When the tree was cut is not nearly as you to use different fuels by looking at the table below. important as when it was split, since seasoning really The fuel cost per heating season was calculated for a begins after splitting. 2,000 square foot home in Maryland using this heating Ask what size truck the dealer delivers in and if it’s truly calculator: www.eia.gov/neic/experts/heatcalc.xls. -

Environmental Considerations of Treated Wood National Park Service – Pacific West Region
Environmental Considerations of Treated Wood National Park Service – Pacific West Region Overview In support of the mission of the National Park Service, making wise decisions about using wood treatments will help protect the natural areas and biodiversity of our parks, and the health of our employees. Preservative-treated wood’s most important benefit is its resistance to water, fungal, and insect damage. Extending the life of wood products reduces the demands on forests for replacement lumber and reduces maintenance and replacement costs. Historic wooden structures that must be repaired with compatible materials or replaced with in-kind materials make durability even more important. Treated woods are nearly impervious to rot and insects, making them good for outdoor use. Wood treated with chromated copper arsenate (CCA) poses certain environmental and health risks, including the leaching of chemicals such as arsenic and chromium into the environment and workers’ risk of exposure to hazardous chemicals. Disposal of treated wood also proves to be an issue, particularly disposal by incineration. Due to these concerns, manufacturers of treated wood and the EPA reached an agreement to end the sale of CCA-treated wood for most lumber products, effective January 1, 2004. The following offers less-toxic alternatives to CCA, handling and use precautions, and other recommendations when considering using treated wood. Due to the toxicity and potential effects on health and the environment, the Presidio Trust implemented a policy on the use of pressure treated lumber. Standard operating procedure now prohibits the use of CCA, ACZA, CZC, ACC, and Pentachlorophenol. All dimensional lumber is now treated with ACQ as an alternative. -

FSA1091 Basics of Heating with Firewood
DIVISION OF AGRICULTURE RESEARCH & EXTENSION Agriculture and Natural Resources University of Arkansas System FSA1091 Basics of Heating with Firewood Sammy Sadaka Introduction Many options of secure, wood combustion Ph.D., P.E. stoves, freplaces, furnaces and boilers Associate Professor Wood heating was the predominant are available in the market. EPA certifed freplaces, furnaces and wood stoves with Extension Engineer means for heating in homes and businesses for several decades until the advent of no visible smoke and 90 percent less iron radiators, forced air furnaces and pollution are among alternatives. Addi- John W. Magugu, Ph.D. improved stoves. More recently, a census tionally, wood fuel users should adhere Professional Assistant by Energy Information Administration, to sustainable wood management and EIA, has placed fuelwood users in the environmental sustainability frameworks. USA at 2.5 million as of 2012. Burning wood has been more common Despite the widespread use of cen- among rural families compared to families tral heating systems, many Arkansans within urban jurisdictions. Burning wood still have freplaces in their homes, with has been further incentivized by more many others actively using wood heating extended utility (power) outages caused systems. A considerable number of by wind, ice and snowstorms. Furthermore, Arkansans tend to depend on wood fuel liquefed petroleum gas, their alternative as a primary source of heating due to fuel, has seen price increases over recent high-energy costs, the existence of high- years. effciency heating apparatuses and Numerous consumers continue to have extended power outages in rural areas. questions related to the use of frewood. An Apart from the usual open freplaces, important question is what type of wood more effcient wood stoves, freplace can be burned for frewood? How to store inserts and furnaces have emerged. -

1. Hand Tools 3. Related Tools 4. Chisels 5. Hammer 6. Saw Terminology 7. Pliers Introduction
1 1. Hand Tools 2. Types 2.1 Hand tools 2.2 Hammer Drill 2.3 Rotary hammer drill 2.4 Cordless drills 2.5 Drill press 2.6 Geared head drill 2.7 Radial arm drill 2.8 Mill drill 3. Related tools 4. Chisels 4.1. Types 4.1.1 Woodworking chisels 4.1.1.1 Lathe tools 4.2 Metalworking chisels 4.2.1 Cold chisel 4.2.2 Hardy chisel 4.3 Stone chisels 4.4 Masonry chisels 4.4.1 Joint chisel 5. Hammer 5.1 Basic design and variations 5.2 The physics of hammering 5.2.1 Hammer as a force amplifier 5.2.2 Effect of the head's mass 5.2.3 Effect of the handle 5.3 War hammers 5.4 Symbolic hammers 6. Saw terminology 6.1 Types of saws 6.1.1 Hand saws 6.1.2. Back saws 6.1.3 Mechanically powered saws 6.1.4. Circular blade saws 6.1.5. Reciprocating blade saws 6.1.6..Continuous band 6.2. Types of saw blades and the cuts they make 6.3. Materials used for saws 7. Pliers Introduction 7.1. Design 7.2.Common types 7.2.1 Gripping pliers (used to improve grip) 7.2 2.Cutting pliers (used to sever or pinch off) 2 7.2.3 Crimping pliers 7.2.4 Rotational pliers 8. Common wrenches / spanners 8.1 Other general wrenches / spanners 8.2. Spe cialized wrenches / spanners 8.3. Spanners in popular culture 9. Hacksaw, surface plate, surface gauge, , vee-block, files 10. -

Harvesting Firewood from Your Woods
Harvesting Firewood from Your Woods TOPICS: n Tree and Forest Biology (page 2) Basic concepts about how trees grow and the characteristics that make trees good or bad for firewood n Planning a Harvest (page 8) Which trees to cut for firewood and how to cut them safely n Processing Trees into Firewood ( p age 14) Techniques for splitting, drying and stacking wood Tree & Forest Biology Cutting trees for firewood requires careful management. The management decisions you make can either improve or harm the long-term health and productivity of your woodlands. Understanding how and where trees grow can aid your deci- sions about which trees to cut, and will lead to improvements in the overall health of your woodlands. Trees have several basic requirements for long-term survival: nutrients, space, water and sunlight. The competition for these resources will determine how well a tree grows and how long it will survive. Tree roots are responsible for the uptake of water and nutrients. The quality and quantity of these nutrients vary depending on the soil. Different trees are adapted to the dif- ferent soil types, from sand to clay. Clay and loam soils hold water and nutrients better than sandy soils. That means trees growing on sandy soils need to be able to grow in low nutrient and low moisture conditions. Each soil type can only support a limited number of trees, based on their size. Trees become stressed when there is too much competition for water and nutrients. To keep your forest healthy, some trees should be removed to make room for others to grow. -

Log Holder This Sturdy Log Holder Makes Stacking Easier and Keeps Your Firewood Organized and Off the Ground
STACK LOGS NEAT AND CLEAN TM Log Holder This sturdy log holder makes stacking easier and keeps your firewood organized and off the ground. Use 2x4 preservative-treated lumber with Simpson Strong-Tie® Rigid Tie® RTC2Z connectors to build this log holder any size you need in less than an hour. Check out our installation and building tip videos at diydoneright.com. TOOLS YOU NEED • Saw • Screw gun • ¼" hex head socket • Tape measure • Clamps 2x4 LUMBER • Framing square Length can vary as needed Connectors are easy to install and strong enough to support even the heaviest loads. RIGID TIE® RTC2Z CONNECTOR INSTALLATION INSTRUCTIONS 1. CUT LUMBER TO SIZE. Since all of your cuts are straight cuts, Simpson Strong-Tie® connectors simplify building with wood. 2. INSTALL CONNECTOR ON VERTICAL POST. Mark height, clamp connector to post and attach with #9x1½" Strong-Drive® SD Connector screws. 3. CONNECT HORIZONTAL RAIL 2x4 LUMBER ON EACH SIDE. Width and height can Use a clamp to vary as needed help hold the wood in the ® seat of the con- Attach Rigid Tie RTC2Z nector during connectors with #9x1½" SD Connector screws installation. For more inspiration, visit diydoneright.com © 2017 Simpson Strong-Tie Company Inc. DIY-CSLGHLDR17 MATERIALS AND CUTTING DIAGRAM TM Log Holder FRONT VIEW SIDE VIEW 30" 47" 8" 50" 15" FOR THIS PROJECT YOU WILL NEED: LUMBER SIMPSON STRONG-TIE® CONNECTORS* FASTENERS (3) – pieces of 2x4 8 ft. lumber (4) – Rigid Tie® RTC2Z connectors (1) – BOX Simpson Strong-Tie® #9x1½" Strong-Drive® SD Connector screws CUT FROM 2x4 x 8' LUMBER (2) – 47" RAILS 47" 47" (1x) (3) – 30" CORNER POSTS 30" 30" 30" (1x) (2) – 8" ENDRAILS & (1) 30" CORNER POST 30" 8" 8" (1x) Use 2x4 8 ft. -

Heating with Firewood
Stewardship Notes Indiana Division of Forestry Heating With Firewood Heating costs seem to rise each year with the ever-increasing demand on our nations' energy sources. Many have found wood is an excellent asset in offsetting yearly heating costs. A warm, glowing fire can be a practical complement to any central heating system and can go a long way toward aiding the pocketbook. Unlike most energy sources, wood is a renewable fuel source. New trees are continually growing. For many people, wood has the advantage of being readily available, easily cut and relatively inexpensive. However, when fuels are burned, pollutants are created; wood is no exception. The EPA has found wood burned for home heating is a leading cause of air pollution in many western cities. Wood stoves sold after July 1, 1988 must meet certain air quality standards. Secondary burning chambers and catalytic combustors are two methods being used by manufacturers to reduce pollutants. Proper stove operation is important to maintain the low emission capabilities of new stoves. The Best Kinds of Firewood Certain species of wood produce more heat than others. The heat a log produces depends on the density, moisture content, resin and ash in the wood. The chart to the right shows the densities and heat values of various tree species common in Indiana. The heat value of hickory is set at 100. The chart lists those woods that burn longest at the top of the list, while those toward the bottom will ignite and burn more quickly. When low-density woods are mixed with high-density woods, the fire will start quickly and burn a long time. -

Sawdust Mulches for Larger Crops, Better Soils
C3DQ5/ Sawdust Mulches for Larger Crops, Better Soils Edited by J. L. Overholser Report No. G-5 July 1955 4- OREGON FOREST PRODUCTS LABORATORY State Board of Forestry and School of Forestry, Oregon State College, Cooperating Corvallis OREGON FOREST PRODUCTS LABORATORY was THEestablished by legislative action in 1941 as a result of active interest of the lumber industry and forestry- minded citizens. It is associated with the State Board of Forestry and the School of Forestry at Oregon State College. An Advisory Committee composed of men from representative interests guides the research program that is directly pointed toward the fuller utilization of Oregon's forest resources. The following men con- stitute the present membership of the Advisory Com- mittee: PAUL PATTERSON, Governor .Chairman ROBERT W. COWLIN Pacific Northwest Forest and Range Experiment Station CHARLES W. Fox Oregon Plywood Interests NILS HULT Willamette Valley Lumbermen's Association CAP.I. A. RASMUSSEN Western Pine Association WILLIAM SWINDELLS West Coast Lumbermen's Association WILLIAM I. WEST . School of Forestry GEORGE SPAUR, State Forester Secretary Sawdust Mulches Used For Larger Crops, Better Soils* edited by J. L. Overholser Large-volume use of sawdust for a mulch on various crops may result in increased yields as shown in field tests at Corvallis during the last 6 years. The Departments of Horticulture, Soils and Bacteriology at Oregon State College have cooperated with the Oregon Forest Products Laboratory in comparing sawdust with straw, leaf mold, and hay as mulches and also where cultivated into the soil for growing strawberries, blueberries, cane fruits, and annuals such as corn, tomatoes, beans, and cabbage. -

Suitability of Sawdust from Three Tropical Timbers for Wood-Cement Composites
Journal of Sustainable Forestry ISSN: 1054-9811 (Print) 1540-756X (Online) Journal homepage: http://www.tandfonline.com/loi/wjsf20 Suitability of sawdust from three tropical timbers for wood-cement composites Charles Antwi-Boasiako, Linda Ofosuhene & Kwadwo B. Boadu To cite this article: Charles Antwi-Boasiako, Linda Ofosuhene & Kwadwo B. Boadu (2018) Suitability of sawdust from three tropical timbers for wood-cement composites, Journal of Sustainable Forestry, 37:4, 414-428, DOI: 10.1080/10549811.2018.1427112 To link to this article: https://doi.org/10.1080/10549811.2018.1427112 Published online: 24 Jan 2018. Submit your article to this journal Article views: 72 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=wjsf20 JOURNAL OF SUSTAINABLE FORESTRY 2018, VOL. 37, NO. 4, 414–428 https://doi.org/10.1080/10549811.2018.1427112 Suitability of sawdust from three tropical timbers for wood- cement composites Charles Antwi-Boasiako, Linda Ofosuhene, and Kwadwo B. Boadu Department of Wood Science and Technology, Faculty of Renewable Natural Resources, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana ABSTRACT KEYWORDS Construction material rising cost and global demand for economically- Cement hydration; sustainable and environmentally-friendly building resources have composite board; inhibitory necessitated the use of sawdust-cement composite. Wood constitu- chemical; total extractive; ents and cement incompatibility hinder its production and need care- wood-cement compatibility ful selection of the timber. Sawdust suitability from Triplochiton scleroxylon, Entandrophragma cylindricum and Klainedoxa gabonensis for wood-cement composite was determined by identifying their chemical constituents and their composites’ physico-mechanical prop- erties. -

Thermal Degradation Behaviors of Sawdust Wood Waste: Pyrolysis Kinetic and Mechanism
Journal(of(Materials(and(( J. Mater. Environ. Sci., 2019, Volume 10, Issue 8, Page 742-755 Environmental(Sciences( ISSN(:(2028;2508( CODEN(:(JMESCN( http://www.jmaterenvironsci.com! Copyright(©(2019,( University(of(Mohammed(Premier(((((( (OuJda(Morocco( Thermal degradation behaviors of sawdust wood waste: pyrolysis kinetic and mechanism MY. Guida1,2,*, S. Lanaya2, Z. Rbihi2, A. Hannioui1,2 1Department of Chemistry and Environment, Faculty of Sciences and Techniques (FST), University of Sultan Moulay Slimane, 23000 Béni-Mellal, Morocco. 2 Organic Chemistry and Analytical Laboratory, Faculty of Sciences and Techniques (FST), University of Sultan Moulay Slimane, 23000 Béni-Mellal, Morocco. Received 25 May 2019, Abstract Revised 22 August 2019, In this study, the thermal behavior of sawdust wood (SW) wastes samples was examined at Accepted 23 August 2019 different heating rates (β) ranging from 2 to 15 °C/min in inert atmosphere using the Keywords technique of thermogravimetric analysis (TGA). As the increment of heating rates, the variations of characteristic parame ters from the TG-DTG curves were determined. Eight Sawdust wood waste ! methods: Friedman (FR), Ozawa-Flynn-Wall (OFW), Vyazovkin (VYA), Kissinger- ! Pyrolysis Akahira-Sunose (KAS), Starink method (ST), Avrami theory (AT), Coats-Redfern and ! Thermogravimetric Criado methods were used in this work to evaluate the kinetic parameters, including analysis apparent activation energy (Ex), reaction order (n) and the appropriate conversion model ! Kinetic analysis f(x). For the range of conversion degree (x) investigated (20 – 80 %), the mean values of parameters apparent activation energy for Sawdust wood waste were 168 KJ/mol, 153 KJ/mol and 164 ! TGA-DTG KJ/mol for FR, OFW and VYA methods respectively. -

E0021 Wood Pellets, an Introduction to Their Production And
Wood Pellets An Introduction to their Production and Use David Jones Mississippi State University David Harper & Adam Taylor University of Tennessee What’s Inside… Page 2 Advantages of Wood Pellets Green, clean, and convenient Introduction Page 3 How Pellets Are Made Turning wood waste into Wood pellets are compressed wood particles that are used as premium fuel fuel. Pellets are already commonly used in some areas of the country, and in other areas they are growing in popularity as Page 4 How Pellets Are Used primary fuel costs increase and concerns about global climate From home heating to change build. industrial boilers This fact sheet is a short introduction to wood pellets and will Page 4 Markets for Pellets be helpful to those who are interested in using or producing Heating up! wood pellet fuel. The advantages of wood pellets are discussed, as well as how pellets are made and used. Wood Pellet Stoves Wood pellet stoves are very efficient and are capable of capturing almost 95 percent of the heat produced from burning the pellets. 1 Wood Pellets: An Introduction Advantages of Wood Pellets Because wood pellets are made from wood, they have the same advantages as wood: local, abundant, renewable, and low-cost. In addition, wood pellets are Both systems require little when wood is burned was very low in moisture (water) and maintenance because the captured from the atmosphere ash content, so they burn hot pellets burn so cleanly. when the wood was grown in and cleanly. Fuel pellets are the tree. This is an important Pellets have a number of limited to 1 percent (premium- advantage, especially in some “environment-friendly” grade) to 3 percent (standard) countries in Europe where attributes.