Feeding Habits of Albacore Thunnus Alalunga in the Transition Region of the Central North Pacific
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fish Bulletin 152. Food Habits of Albacore, Bluefin Tuna, and Bonito in California Waters
UC San Diego Fish Bulletin Title Fish Bulletin 152. Food Habits of Albacore, Bluefin Tuna, and Bonito In California Waters Permalink https://escholarship.org/uc/item/7t5868rd Authors Pinkas, Leo Oliphant, Malcolm S Iverson, Ingrid L.K. Publication Date 1970-06-01 eScholarship.org Powered by the California Digital Library University of California STATE OF CALIFORNIA THE RESOURCES AGENCY DEPARTMENT OF FISH AND GAME FISH BULLETIN 152 Food Habits of Albacore, Bluefin Tuna, and Bonito In California Waters By Leo Pinkas , Malcolm S. Oliphant, and Ingrid L. K. Iverson 1971 1 2 ABSTRACT The authors investigated food habits of albacore, Thunnus alalunga, bluefin tuna, Thunnus thynnus, and bonito, Sarda chiliensis, in the eastern North Pacific Ocean during 1968 and 1969. While most stomach samples came from fish caught commercially off southern California and Baja California, some came from fish taken in central Califor- nia, Oregon, and Washington waters. Standard procedures included enumeration of food items, volumetric analysis, and measure of frequency of occur- rence. The authors identified the majority of forage organisms to the specific level through usual taxonomic methods for whole animals. Identification of partially digested animals was accomplished through the use of otoliths for fish, beaks for cephalopods, and the exoskeleton for invertebrates. A pictorial guide to beaks of certain eastern Pacific cephalopods was prepared and proved helpful in identifying stomach contents. This guide is presented in this publication. The study indicates the prominent forage for bluefin tuna, bonito, and albacore in California waters is the northern anchovy, Engraulis mordax. 3 ACKNOWLEDGMENTS The Food Habits Study of Organisms of the California Current System, (Project 6–7-R), was an investigation estab- lished under contract between the U.S. -

16. Bering Sea and Aleutian Islands Squids
16. Bering Sea and Aleutian Islands Squids Olav A. Ormseth and Elaina Jorgenson NMFS Alaska Fisheries Science Center Executive Summary Summary of Major Changes Changes in the input data: Total catch data for Bering Sea and Aleutian Islands (BSAI) squids is updated with 2006 and partial 2007 data. Changes in assessment methodology: There are no changes in the assessment methodology. Changes in assessment results: There are no changes in assessment results because BSAI squids remain in Tier 6, as they have for the past several years. The recommended allowable biological catch (ABC) for squids in 2008 and 2009 is calculated as 0.75 multiplied by the average catch from 1978-1995, or 1,970 t; the recommended overfishing level (OFL) for squid in the years 2008-2009 is calculated as the average catch from 1978- 1995, or 2,624 t. We continue to lack reliable squid biomass information that would allow a Tier 5 or higher assessment. 2008-2009 Tier 6 harvest specifications for BSAI squids 2008-2009 ABC 1,970 t 2008-2009 OFL 2,624 t Responses to SSC Comments From the December 2006 SSC minutes: 1) For squid, it would be useful to see an analysis of the spatial distribution of catches for consideration in devising alternative tier 6 approaches. Response: Spatial analyses for 2000-2006 have not been completed, but we anticipate that this will be done in 2008. Introduction Description, scientific names, and general distribution Squids are marine molluscs in the class Cephalopoda (Group Decapodiformes). Squids are considered highly specialized and organized molluscs, with only a vestigial mollusc shell remaining as an internal plate called the pen or gladius. -

During 1979-1997
ICES CM/1988M:39 (Poster) 38.13 4 Interannual Variability in the Neon Flying Squid Abundance and Oceanographic Conditions in the Central North Pacific Ocean during 1979-1997 A. Yatsu*, J. Mori*, T. Watanabe*, T. Meguro", Y. Kamei", and Y. Sakurai" Abstract The neon flying squid, Ommastrephes bartrami, was the target species of the Japanese squid driftnet fishery in the Central North Pacific Ocean during 1979- 1992. Interannual variation in the neon flying squid catch-per-unit-effort (CPUE) in this fishery was highly correlated with that of the Hokkaido University's research driftnet surveys since 1979 along 175' 307Ein July which coincided with the peak of the commercial fishery. While productivity in the northern North Pacific Ocean was high during the 1970's and declining to the present days, the research net CPUE of 0. bartrami was higher in 1979 and in 1994-97 than in other years, suggesting effect of fishing on the stock abundance. The distributions and CPUE variability in 0. bartarmi were also affected by water temperature and salinity structures around the Subarctic Boundary. During the intensive driftnet fishing, three squid species (0. bartrami, Gonatopsis borealis and Onchoteuthis borealijaponica) may have, to some extent, filled throphic niche that was occupied by pelagic fishes. National Research Institute of Far Sear Fisheries, Shimizu 424-8633, Japan " Faculty of Fisheries, Hokknido University, Hakodate 041-8611, Japan Introduction end of 1992 according to the bycatch problem. Since 1993, fishing mortality The neon flying squid, Ommastrephes of the autumn cohort has been derived bartrami, is one of the most dominant only by jig fishing whose annual catch nekton in the epipelagic subtropical and has been less than approximately 10,000 subpolar waters of the world oceans, and tons for this cohort. -

SYNOPSIS of BIOLOGICAL DATA on SPECIES of the GENUS Thunnus (Sensu Lato) (SOUTH AFRICA)
Species Synopsis No, 19 FAO Fisheries Biology Synopsis No, 62 FIb/SG2 (Distribution restricted) SAST - Tuna SYNOPSIS OF BIOLOGICAL DATA ON SPECIES OF THE GENUS Thunnus (Sensu lato) (SOUTH AFRICA) Exposé synoptique sur la biologie des espèces du genre Thunnus (Sensu lato) (Afrique du Sud) Sinopsis sobre la biologia de las especies del género Thunnus (Sensu lato) (Sudfrica) Prepared by F, H, TALBOT and M, J, PENRITH South African Museum Cape Town, South Africa FISHERIES DIVISION, BIOLOGY BRANCH FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 1963 bU 8 FIb/S62 Thunnus alalunga 1:1 Thunnus alalunga IDENTITY species in having a clear white edge to the caudal. 1. 1Taxonomy Liver: Center of the three lobeb largest. Densely striated with surface veins ventrally, i. 1, 1Definition-ih similar to bluefin tuna. A Thunnus with liver densely striated with Swim bladder: Wide, and running nearly veins ventrally;cutaneous blood vessels pas- the full length of the body cavity, with marked sing through the myotome of the 5th vertebra; pit anteriorly, not divided into two by a con- with pectoral long, at least reaching beyond the nective tissue wall as found in the bigeye tuna, 2nd dorsal; and a total count of 27 to 30 gill- but may be slightly cleft anteriorly (three rakers on the first arch. specimens dissected). 1. 1. 2 Description-2/ 1. 2 Nomenclature Torpedo-shaped body, less deep and less 1. 2. 1Valid scientific name compressed than most tunas. Thunnus alalunga (Bonnaterre) Proportions: (expressed as a percentage of fork length).Head, 29 to 30;depth, 25 to 1.2.2 Synonyms-li 27;eye, 5.3 to 5.7; maxilla, 10 to 12; pec- toral length, 40 to 42;first dorsal height,11 Scomber alalunga Bonnaterre, 1788, p. -

Visual Adaptations in Crustaceans: Chromatic, Developmental, and Temporal Aspects
FAU Institutional Repository http://purl.fcla.edu/fau/fauir This paper was submitted by the faculty of FAU’s Harbor Branch Oceanographic Institute. Notice: ©2003 Springer‐Verlag. This manuscript is an author version with the final publication available at http://www.springerlink.com and may be cited as: Marshall, N. J., Cronin, T. W., & Frank, T. M. (2003). Visual Adaptations in Crustaceans: Chromatic, Developmental, and Temporal Aspects. In S. P. Collin & N. J. Marshall (Eds.), Sensory Processing in Aquatic Environments. (pp. 343‐372). Berlin: Springer‐Verlag. doi: 10.1007/978‐0‐387‐22628‐6_18 18 Visual Adaptations in Crustaceans: Chromatic, Developmental, and Temporal Aspects N. Justin Marshall, Thomas W. Cronin, and Tamara M. Frank Abstract Crustaceans possess a huge variety of body plans and inhabit most regions of Earth, specializing in the aquatic realm. Their diversity of form and living space has resulted in equally diverse eye designs. This chapter reviews the latest state of knowledge in crustacean vision concentrating on three areas: spectral sensitivities, ontogenetic development of spectral sen sitivity, and the temporal properties of photoreceptors from different environments. Visual ecology is a binding element of the chapter and within this framework the astonishing variety of stomatopod (mantis shrimp) spectral sensitivities and the environmental pressures molding them are examined in some detail. The quantity and spectral content of light changes dra matically with depth and water type and, as might be expected, many adaptations in crustacean photoreceptor design are related to this governing environmental factor. Spectral and temporal tuning may be more influenced by bioluminescence in the deep ocean, and the spectral quality of light at dawn and dusk is probably a critical feature in the visual worlds of many shallow-water crustaceans. -

Redacted for Privacy William G
AN ABSTRACT OF THE THESIS OF Elizabeth H. Sinclair for the degree of Master of Science in Oceanography presented on December 16, 1988. Title: Feeding Habits of Northern Fur Seals (Callorhinus ursinus) in the Eastern Bering Sea Abstract approved: Redacted for privacy William G. Pearcy This study was conducted to determine the composition and size of prey consumed by northern fur seals (Callorhinus ursinus) in the eastern Bering Sea. Eighty three northern fur seals were collected in the summer and fall of 1981, 1982, and 1985 forexamination of gastrointestinal contents. A total of 139 midwater and bottom trawls were collected to determine the availability of potential prey. Analysis of trawls confirmed that seals are size-selective midwater feeders during their breeding and haul-out season in the eastern Bering Sea. Juvenile walleye pollock and gonatid squid, 5-20cm in body length, were the primary prey, but seal prey varied among years and between nearshore and pelagic sample locations. Interannual variation in body sizes of walleye pollock consumed by seals was related to pollock year class strength. The identification of pollock and gonatid squid as primary fur seal prey in the eastern Bering Sea was consistent with previous reports. However, Pacific herring and capelin, previously considered important fur seal prey werE absent in this study. Feeding Habits of Northern Fur Seals (Callorhinus ursinus) in the Eastern Bering Sea by Elizabeth Hacker Sinclair A THESIS submitted to Oregon State University in partial fulfillment of the requirements for the degree of Master of Science Completed December 16, 1988 Commencement June 1989 APPROVED: Redacted for privacy Professor of Oceanography in char.4f major Redacted for privacy Dean of C./lege of Ocean. -

Journal of Natural History
This article was downloaded by:[Smithsonian Trpcl Res Inst] [Smithsonian Trpcl Res Inst] On: 24 May 2007 Access Details: [subscription number 777121079] Publisher: Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK Journal of Natural History Publication details, including instructions for authors and subscription information: http://www.informaworld.com/smpp/title~content=t713192031 Extended parental care in two endobenthic amphipods M. Thiel a; S. Sampson a; L. Watling a a Darling Marine Center, University of Maine. Walpole, ME. USA To cite this Article: Thiel, M., Sampson, S. and Watling, L. , 'Extended parental care in two endobenthic amphipods', Journal of Natural History, 31:5, 713 - 725 To link to this article: DOI: 10.1080/00222939700770351 URL: http://dx.doi.org/10.1080/00222939700770351 PLEASE SCROLL DOWN FOR ARTICLE Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf This article maybe used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden. The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material. -
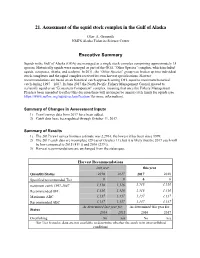
Assessment of the Squid Stock Complex in the Gulf of Alaska
21. Assessment of the squid stock complex in the Gulf of Alaska Olav A. Ormseth NMFS Alaska Fisheries Science Center Executive Summary Squids in the Gulf of Alaska (GOA) are managed as a single stock complex comprising approximately 15 species. Historically squids were managed as part of the GOA “Other Species” complex, which included squids, octopuses, sharks, and sculpins. In 2011, the “Other Species” group was broken up into individual stock complexes and the squid complex received its own harvest specifications. Harvest recommendations are based on an historical catch approach setting OFL equal to maximum historical catch during 1997 – 2007. In June 2017 the North Pacific Fishery Management Council moved to reclassify squid as an “Ecosystem Component” complex, meaning that once the Fishery Management Plan has been amended to reflect this decision there will no longer be annual catch limits for squids (see https://www.npfmc.org/squid-reclassification/ for more information). Summary of Changes in Assessment Inputs 1) Trawl survey data from 2017 have been added. 2) Catch data have been updated through October 11, 2017. Summary of Results 1) The 2017 trawl survey biomass estimate was 2,296 t, the lowest it has been since 1999. 2) The 2017 catch data are incomplete (29 t as of October 11), but it is likely that the 2017 catch will be low compared to 2015 (411 t) and 2016 (239 t). 3) Harvest recommendations are unchanged from the status quo. Harvest Recommendations last year this year Quantity/Status 2016 2017 2017 2018 Specified/recommended -

8.1 the Significance of Ocean Deoxygenation for Mesopelagic Communities Brad A
8.1 The significance of ocean deoxygenation for mesopelagic communities Brad A. Seibel and Karen F. Wishner 8.1 The significance of ocean deoxygenation for mesopelagic communities Brad A. Seibel1 and Karen F. Wishner2 1College of Marine Science, University of South Florida, Florida, USA. Email: [email protected] 2Graduate School of Oceanography, University of Rhode Island, Kingston, Rhode island, USA. Email: [email protected] Summary • Mesopelagic community structure is directly dependent on the availability of oxygen for aerobic metabolism. Diversity, abundance, distribution and composition of mesopelagic species are all influenced by variations in oxygen at both large and small scales. • Ocean deoxygenation will decrease the minimum oxygen content in the mesopelagic zone and cause oxyclines to shift vertically (i.e. expansion of the oxygen minimum zone (OMZ) core) in the water column. • A species’ ability to extract oxygen from sea water has evolved to meet specific oxygen demand. As a result, species do not have excess capacity, nor do they live in environments with excess oxygen relative to their evolved capacity; thus, they are susceptible to reductions in oxygen partial pressure and increasing temperature (which elevates metabolic demand). • Changes in temperature and oxygen profiles within the water column may therefore decouple or enhance competition among different mesopelagic zooplankton species and the larger predators that forage on them at depth by changing zooplankton abundances, distributions, and the depth of layers, and altering species composition and diversity. The biogeochemical cycles (i.e. the biological pump and microbial assemblages) that rely on the mesopelagic zooplankton community will be substantially altered. SECTION 8.1 SECTION Ocean deoxygenation: Everyone’s problem 265 8.1 The significance of ocean deoxygenation for mesopelagic communities Ocean hypoxia effect Potential consequences Decreasing oxygen partial pressure (PO2) in any • Reduced capacity for prey capture and predator habitat will reduce aerobic metabolic performance evasion. -

Patterns in Micronekton Diversity Across the North Pacific Subtropical Gyre Observed from the Diet of Longnose Lancetfish (Alepisaurus Ferox)
Author’s Accepted Manuscript Patterns in micronekton diversity across the North Pacific Subtropical Gyre observed from the diet of longnose lancetfish (Alepisaurus ferox) Elan J. Portner, Jeffrey J. Polovina, C. Anela Choy www.elsevier.com PII: S0967-0637(16)30357-0 DOI: http://dx.doi.org/10.1016/j.dsr.2017.04.013 Reference: DSRI2784 To appear in: Deep-Sea Research Part I Received date: 28 October 2016 Revised date: 7 March 2017 Accepted date: 18 April 2017 Cite this article as: Elan J. Portner, Jeffrey J. Polovina and C. Anela Choy, Patterns in micronekton diversity across the North Pacific Subtropical Gyre observed from the diet of longnose lancetfish (Alepisaurus ferox) , Deep-Sea Research Part I, http://dx.doi.org/10.1016/j.dsr.2017.04.013 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting galley proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. Patterns in micronekton diversity across the North Pacific Subtropical Gyre observed from the diet of longnose lancetfish (Alepisaurus ferox) Elan J. Portnera*, Jeffrey J. Polovinab, C. Anela Choyc aHopkins Marine Station, Stanford University, 120 Ocean View Blvd., Pacific Grove, CA 93950, USA bNOAA Pacific Islands Fisheries Science Center 1845 Wasp Blvd., Honolulu, HI 96818, USA cMonterey Bay Aquarium Research Institute 7700 Sandholdt Road, Moss Landing, CA 95039, USA [email protected] [email protected] [email protected] *Corresponding author Abstract: We examined the diet of a common midwater predator, the longnose lancetfish (Alepisaurus ferox, n= 1371), with respect to fork length, season, and capture location within the North Pacific Subtropical Gyre (NPSG). -

Life History of the Neon Flying Squid: Effect of the Oceanographic Regime
Vol. 378: 1–11, 2009 MARINE ECOLOGY PROGRESS SERIES Published March 12 doi: 10.3354/meps07873 Mar Ecol Prog Ser OPENPEN ACCESSCCESS FEATURE ARTICLE Life history of the neon flying squid: effect of the oceanographic regime in the North Pacific Ocean Taro Ichii1,*, Kedarnath Mahapatra2, Mitsuo Sakai1, Yoshihiro Okada3 1National Research Institute of Far Seas Fisheries, 2-12-4 Fukuura, Kanazawa, Yokohama-city, Kanagawa 236-8648, Japan 2Tokai University Frontier Ocean Research Center (T-FORCE), 3-20-1 Orido, Shimizu-ward, Shizuoka-city, Shizuoka 424-8610, Japan 3School of Marine Science and Technology, Tokai University, 3-20-1 Orido, Shimizu-ward, Shizuoka-city, Shizuoka 424-8610, Japan ABSTRACT: The North Pacific Ocean population of the neon flying squid Ommastrephes bartramii, which un- dertakes seasonal north–south migrations, consists of autumn and winter–spring spawning cohorts. We ex- amined life history differences between the 2 cohorts in relation to the oceanographic environment. The differ- ences could be explained by seasonal north–south movements of the following 2 oceanographic zones: (1) the optimum spawning zone defined by sea surface temperatures; and (2) the food-rich zone defined by the position of the transition zone chlorophyll front (TZCF). The 2 cohorts use the food-rich zone in different phases of their life cycles. The spawning grounds for the au- Hatchling of the neon flying squid Ommastrephes bartramii tumn cohort occur within the subtropical frontal zone Photo: M. Sakai (STFZ), characterized by enhanced productivity in win- ter due to its proximity to the TZCF, whereas the spawning grounds for the winter–spring cohort occur within the subtropical domain, which is less productive. -

Amphipods and Euphausiids in the Summer of the Western North Pacific
Vertical Distribution, Community Structure, and Active Carbon Flux of Two Macrozooplankton Taxa : Amphipods and Title Euphausiids in the Summer of the Western North Pacific Author(s) Hanamiya, Yurika; Murase, Hiroto; Matsuno, Kohei; Yamaguchi, Atsushi Citation 北海道大学水産科学研究彙報, 70(1), 77-89 Issue Date 2020-08-24 DOI 10.14943/bull.fish.70.1.77 Doc URL http://hdl.handle.net/2115/79118 Type bulletin (article) File Information bull.fish.70.1.77.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP Bull. Fish. Sci. Hokkaido Univ. HANAMIYA et al. : Vertical distribution of amphipods and euphausiids 70(1), 77-89, 2020. DOI 10.14943/bull.fish.70.1.77 Vertical Distribution, Community Structure, and Active Carbon Flux of Two Macrozooplankton Taxa : Amphipods and Euphausiids in the Summer of the Western North Pacific Yurika Hanamiya1,2), Hiroto Murase3,4), Kohei Matsuno1,5) and Atsushi Yamaguchi1,5) (Received 24 April 2020, Accepted 11 May 2020) Abstract This study conducted diel vertical migration and active migration flux estimation of macrozooplanktonic amphipods and euphausiids at 0-250 m water column of the three stations in the western North Pacific during summer. For amphipods, 25 spe- −2 cies belonging to 17 genera were identified. Their standing stock was 60-574 ind. m during the daytime and 35-5,228 ind. −2 m at night-time. For euphausiids, 19 species belonging to 7 genera were identified. The standing stock of euphausiids was −2 −2 80-382 ind. m and 286-2,156 ind. m during the day and at night, respectively. Feeding impacts during the night were esti- −2 −1 −2 −1 mated to be 0.19-11.76 mg C m day (amphipods) and 5.12-16.42 mg C m day (euphausiids).