“From Raw Material to Next Generation Advanced Batteries”
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Direct Preparation of Some Organolithium Compounds from Lithium and RX Compounds Katashi Oita Iowa State College
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1955 Direct preparation of some organolithium compounds from lithium and RX compounds Katashi Oita Iowa State College Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Oita, Katashi, "Direct preparation of some organolithium compounds from lithium and RX compounds " (1955). Retrospective Theses and Dissertations. 14262. https://lib.dr.iastate.edu/rtd/14262 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand comer and continuing from left to right in equal sections with small overiaps. -

Dilithium Sequestrene As Ananticoagulant
J Clin Pathol: first published as 10.1136/jcp.12.3.254 on 1 May 1959. Downloaded from J. clin. Path. (1959), 12, 254. DILITHIUM SEQUESTRENE AS AN ANTICOAGULANT BY L. S. SACKER,* K. E. SAUNDERS,t BERYL PAGE, AND MARGARET GOODFELLOW From the Departments of Pathology, Dulwich and Lewisham Hospitals, London (RECEIVED FOR PUBLICATION SEPTEMBER 20, 1958) The variety of different anticoagulants used for Ethylene diamine tetra-acetic acid (E.D.T.A.), 29.2 blood samples has increased, but there is still need g., and 7.4 g. of lithium carbonate were intimately for a suitable anticoagulant for the routine mixed. Then 200 ml. of water was added and solution estimation of sodium and potassium by flame took place with vigorous evolution of carbon dioxide. photometry. So far the only satisfactory substance for this purpose has been the expensive calcium Method 2 heparin (King and Wootton, 1956). Disodium sequestrene (Proescher, 1951 ; Hadley E.D.T.A., 29.2 g., was dissolved in 200 ml. of normal and Larson, 1953) and dipotassium sequestrene lithium hydroxide solution (41.96 g. LiOH.H20 per are the most valuable of litre). (Hadley and Weiss, 1955) In both cases the resulting solution contained the the recently introduced anticoagulants for routine dilithium salt. haematological procedures because they preserve Dilithium sequestrene was obtained from these morphology of leucocytes for short periods the solutions after filtration to remove a small quantity copyright. better than the ammonium and potassium oxalate of amorphous debris, by precipitation on the addition mixture or heparin, and they prevent platelets of an equal volume of absolute methyl alcohol and clumping and preserve them. -

Electrolyte Stability and Discharge Products of an Ionic-Liquid-Based Li-O2 Battery Revealed by Soft X-Ray Emission Spectroscopy
Lawrence Berkeley National Laboratory Recent Work Title Electrolyte Stability and Discharge Products of an Ionic-Liquid-Based Li-O2 Battery Revealed by Soft X-Ray Emission Spectroscopy Permalink https://escholarship.org/uc/item/6gd3f1x1 Journal Journal of Physical Chemistry C, 123(51) ISSN 1932-7447 Authors León, A Fiedler, A Blum, M et al. Publication Date 2019-12-26 DOI 10.1021/acs.jpcc.9b08777 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Electrolyte Stability and Discharge Products of an Ionic- Liquid-based Li-O2 Battery Revealed by Soft X-Ray Emission Spectroscopy Aline Léon*1, Andy Fiedler2, Monika Blum3,4, Wanli Yang4, Marcus Bär5,6,7, Frieder Scheiba2, Helmut Ehrenberg2, Clemens Heske1,3,8, and Lothar Weinhardt1,3,8 1) Institute for Photon Science and Synchrotron Radiation (IPS), Karlsruhe Institute of Technology (KIT), Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany 2) Institute for Applied Materials (IAM), Karlsruhe Institute of Technology (KIT), Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany 3) Department of Chemistry and Biochemistry, University of Nevada, Las Vegas (UNLV), NV 89154-4003, USA 4) Advanced Light Source (ALS), Lawrence Berkeley National Laboratory, Berkeley, California 94720, United States 5) Department of Interface Design, Helmholtz-Zentrum Berlin für Materialien und Energie GmbH (HZB), Albert-Einstein-Str. 15, 12489 Berlin, Germany 6) Helmholtz-Institute Erlangen-Nürnberg for Renewable Energy (HI ERN), Albert-Einstein-Str. 15, 12489 Berlin, Germany 1 7) Department of Chemistry and Pharmacy, Friedrich-Alexander- Universität Erlangen-Nürnberg, Egerlandstr. 3, 91058 Erlangen, Erlangen, Germany 8) Institute for Chemical Technology and Polymer Chemistry (ITCP), Karlsruhe Institute of Technology (KIT), Engesserstr. -

Common Name: LITHIUM ALUMINUM HYDRIDE HAZARD
Common Name: LITHIUM ALUMINUM HYDRIDE CAS Number: 16853-85-3 RTK Substance number: 1121 DOT Number: UN 1410 Date: April 1986 Revision: November 1999 ----------------------------------------------------------------------- ----------------------------------------------------------------------- HAZARD SUMMARY * Lithium Aluminum Hydride can affect you when * Exposure to hazardous substances should be routinely breathed in. evaluated. This may include collecting personal and area * Contact can cause severe skin and eye irritation and burns. air samples. You can obtain copies of sampling results * Breathing Lithium Aluminum Hydride can irritate the from your employer. You have a legal right to this nose and throat. information under OSHA 1910.1020. * Breathing Lithium Aluminum Hydride can irritate the * If you think you are experiencing any work-related health lungs causing coughing and/or shortness of breath. Higher problems, see a doctor trained to recognize occupational exposures can cause a build-up of fluid in the lungs diseases. Take this Fact Sheet with you. (pulmonary edema), a medical emergency, with severe shortness of breath. WORKPLACE EXPOSURE LIMITS * Exposure can cause loss of appetite, nausea, vomiting, The following exposure limits are for Aluminum pyro powders diarrhea and abdominal pain. (measured as Aluminum): * Lithium Aluminum Hydride can cause headache, muscle weakness, loss of coordination, confusion, seizures and NIOSH: The recommended airborne exposure limit is coma. 5 mg/m3 averaged over a 10-hour workshift. * High exposure can affect the thyroid gland function resulting in an enlarged thyroid (goiter). ACGIH: The recommended airborne exposure limit is * Lithium Aluminum Hydride may damage the kidneys. 5 mg/m3 averaged over an 8-hour workshift. * Lithium Aluminum Hydride is a REACTIVE CHEMICAL and an EXPLOSION HAZARD. -

Lithium Aluminum Hydride
Lithium aluminum hydride sc-215254 Material Safety Data Sheet Hazard Alert Code EXTREME HIGH MODERATE LOW Key: Section 1 - CHEMICAL PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME Lithium aluminum hydride STATEMENT OF HAZARDOUS NATURE CONSIDERED A HAZARDOUS SUBSTANCE ACCORDING TO OSHA 29 CFR 1910.1200. NFPA FLAMMABILITY4 HEALTH3 HAZARD INSTABILITY2 W SUPPLIER Santa Cruz Biotechnology, Inc. 2145 Delaware Avenue Santa Cruz, California 95060 800.457.3801 or 831.457.3800 EMERGENCY ChemWatch Within the US & Canada: 877-715-9305 Outside the US & Canada: +800 2436 2255 (1-800-CHEMCALL) or call +613 9573 3112 SYNONYMS Li-Al-H4, Al-H4-Li, "lithium aluminum hydride", "lithium aluminum tetrahydride", "lithium aluminium tetrahydride", "lithium tetrahydroaluminate", "aluminate, tetrahydro-, lithium", "lithium aluminohydride", "aluminum lithium hydride", "lithium alanate", LAH Section 2 - HAZARDS IDENTIFICATION CHEMWATCH HAZARD RATINGS Min Max Flammability 4 Toxicity 2 Body Contact 4 Min/Nil=0 Low=1 Reactivity 2 Moderate=2 High=3 Chronic 2 Extreme=4 CANADIAN WHMIS SYMBOLS 1 of 10 CANADIAN WHMIS CLASSIFICATION CAS 16853-85-3Lithium tetrahydroaluminate E-Corrosive Material EMERGENCY OVERVIEW RISK Causes severe burns. Risk of serious damage to eyes. Reacts violently with water liberating extremely flammable gases. Extremely flammable. POTENTIAL HEALTH EFFECTS ACUTE HEALTH EFFECTS SWALLOWED ■ The material can produce severe chemical burns within the oral cavity and gastrointestinal tract following ingestion. ■ Ingestion of alkaline corrosives may produce burns around the mouth, ulcerations and swellings of the mucous membranes, profuse saliva production, with an inability to speak or swallow. Both the oesophagus and stomach may experience burning pain; vomiting and diarrhoea may follow. ■ Accidental ingestion of the material may be damaging to the health of the individual. -

Chemical List
1 EXHIBIT 1 2 CHEMICAL CLASSIFICATION LIST 3 4 1. Pyrophoric Chemicals 5 1.1. Aluminum alkyls: R3Al, R2AlCl, RAlCl2 6 Examples: Et3Al, Et2AlCl, EtAlCl2, Me3Al, Diethylethoxyaluminium 7 1.2. Grignard Reagents: RMgX (R=alkyl, aryl, vinyl X=halogen) 8 1.3. Lithium Reagents: RLi (R = alkyls, aryls, vinyls) 9 Examples: Butyllithium, Isobutyllithium, sec-Butyllithium, tert-Butyllithium, 10 Ethyllithium, Isopropyllithium, Methyllithium, (Trimethylsilyl)methyllithium, 11 Phenyllithium, 2-Thienyllithium, Vinyllithium, Lithium acetylide ethylenediamine 12 complex, Lithium (trimethylsilyl)acetylide, Lithium phenylacetylide 13 1.4. Zinc Alkyl Reagents: RZnX, R2Zn 14 Examples: Et2Zn 15 1.5. Metal carbonyls: Lithium carbonyl, Nickel tetracarbonyl, Dicobalt octacarbonyl 16 1.6. Metal powders (finely divided): Bismuth, Calcium, Cobalt, Hafnium, Iron, 17 Magnesium, Titanium, Uranium, Zinc, Zirconium 18 1.7. Low Valent Metals: Titanium dichloride 19 1.8. Metal hydrides: Potassium Hydride, Sodium hydride, Lithium Aluminum Hydride, 20 Diethylaluminium hydride, Diisobutylaluminum hydride 21 1.9. Nonmetal hydrides: Arsine, Boranes, Diethylarsine, diethylphosphine, Germane, 22 Phosphine, phenylphosphine, Silane, Methanetellurol (CH3TeH) 23 1.10. Non-metal alkyls: R3B, R3P, R3As; Tributylphosphine, Dichloro(methyl)silane 24 1.11. Used hydrogenation catalysts: Raney nickel, Palladium, Platinum 25 1.12. Activated Copper fuel cell catalysts, e.g. Cu/ZnO/Al2O3 26 1.13. Finely Divided Sulfides: Iron Sulfides (FeS, FeS2, Fe3S4), and Potassium Sulfide 27 (K2S) 28 REFERRAL -

Synthesis of Lithium Peroxide from Hydrogen Peroxide and Lithium Hydroxide in Aqueous-Organic Medium: Wasteless Technology
MATEC Web of Conferences 96, 00004 (2017) DOI: 10.1051/ matecconf/20179600004 REE-2016 Synthesis of Lithium Peroxide from Hydrogen Peroxide and Lithium Hydroxide in Aqueous-Organic Medium: Wasteless Technology Roman Nefedov* and Yury Ferapontov Tambov State Technical University, Tambov, Russia Abstract. Lithium is a rare element, and widely used in manufacturing, electronics, medicine, and etc. One of the important lithium compounds is lithium peroxide. It is a component of regenerating products to protect the human respiratory system from damaging factors of chemical and biological nature. This paper describes the methods of obtaining the lithium peroxide. All industrial techniques for the synthesis of lithium peroxide are presented. A critical assessment of these techniques is given. A new wasteless synthesis technology of lithium peroxide from Hydrogen Peroxide and Lithium Hydroxide in Aqueous-Organic Medium is presented. This technology allows obtaining the pure product containing Li2O2 up to 94 %. The possibility of the solvent regeneration by the anhydrous lithium hydroxide has been shown. The yield of the lithium – 98 % and a significant reduction in solvent consumption per unit of finished product has been obtained. 1 Introduction The use of ethanol is not a prerequisite for obtaining lithium peroxide, although it is beneficial in terms of that The inorganic peroxide compounds based on rare, lithium peroxide contained in it unlike other organic radioactive, and especially alkali metals have a wide substances practically insoluble (dissolved in methanol range of industrial applications (e.g., energy storage and about 3.5 g/L Li2O2. Ethanol can be replaced by other oxygen source, etc.) [1, 2]. organic solvents (methanol, pyridine, propanol, kenotic An identifying characteristic of inorganic peroxide and others [12, 14]). -

United States Patent (19) 11 Patent Number: 5,993,767 Willmann Et Al
USOO5993767A United States Patent (19) 11 Patent Number: 5,993,767 Willmann et al. (45) Date of Patent: Nov.30, 1999 54). SOLVATE OF LITHIUM 5,378,445 1/1995 Salmon et al. .......................... 423/301 HEXAFLUOROPHOSPHATE AND PYRIDINE, 5,616,636 4/1997 Avar et al. ................................ 546/22 ITS PREPARATION AND PREPARATION FOREIGN PATENT DOCUMENTS PROCESS FOR LITHIUM HEXAFLUOROPHOSPHATE USING SAD 20 26 110 12/1970 Germany. SOLVATE OTHER PUBLICATIONS 75 Inventors: Patrick Willmann, Montgiscard; Chemical Abstracts, vol. 100, No. 21, 21 Mai 1984, Colum Régine Naejus, Tours; Robert bus, Ohio, US; abstract no 174608c, Mohamed, K. er at..: Coudert, Notre Dame D'Oe; Daniel “pyridinium poly (hydrogen fluoride)—a reagent for the Lemordant, Orsay, all of France preparation of hexafluorophosphates' XP002024671. 73 Assignee: Centre National D’Etudes Spatiales, Primary Examiner Wayne Langel France Attorney, Agent, or Firm-Burns, Doane, Swecker & Mathis, L.L.P. 21 Appl. No.: 09/000,232 57 ABSTRACT 22 PCT Filed: Jun. 18, 1997 The invention relates to a lithium hexafluorophosphate Sol 86 PCT No.: PCT/FR97/01097 Vate usable for the preparation of high purity lithium S371 Date: Jan. 23, 1998 hexafluorophosphate. S 102(e) Date: Jan. 23, 1998 This solvate of lithium hexafluorophosphate and pyridine 87 PCT Pub. No.: WO97/48709 complies with the formula: PCT Pub. Date: Dec. 24, 1997 30 Foreign Application Priority Data and is prepared by a proceSS comprising the following Stages: Jun. 19, 1996 FR France ................................... 96 O7623 a) preparation of pyridinium hexafluorophosphate of for 511 Int. Cl.CI. ......................... C01B 25/105/10; CO7D 213/20; mula CH-NHPF by the neutralization of hexafluoro CO7F 9/28 phosphoric acid HPF with pyridine and 52 U.S. -

Expansion of Domestic Production of Lithium Carbonate and Lithium Hydroxide to Supply US Battery Industry
Expansion of Domestic Production of Lithium Carbonate and Lithium Hydroxide to Supply US Battery Industry Austin Devaney Jeff Davis Chemetall Foote Corp May 16, 2012 Project ID# ARRAVT010 This presentation does not contain any proprietary, confidential, or otherwise restricted information Overview Expand Lithium Raw Material Base in US Timeline Barriers Start Date: April 14, 2010 Geothermal Resource Strength and Viability of End Date: December, 2013 Geothermal resource Partners Budget Engineering: BE&K (a KBR DOE Share - $28.4 million company) Rockwood Share - $46.0 Environmental Assessment: million Nevada Bureau Land Mgmt Relevance: Domestic Source of Strategic Materials • Objectives – Expand domestic lithium carbonate and lithium hydroxide production to supply the US electric drive automotive market. – Deliver high quality lithium products to battery component manufacturers to produce high quality lithium ion batteries. – Create construction jobs over three years in the US and permanent jobs for production of lithium raw materials. – Stimulate the US economy with worthwhile long term benefits that will support the conversion to electric drive mobility. Relevance: Domestic Source of Strategic Materials • Milestones – Deliver battery grade lithium products to the DOE and component manufacturers in 2012 from this project. – Maintain the long term viability of domestic production of lithium raw materials by lowering operating cost and at the same time reducing fossil fuel based energy consumption. – Job Creation throughout 2010-2013 for -

United States Patent Office Patented Jan
3,711,407 United States Patent Office Patented Jan. 16, 1973 2 in the finished grease or during the neutralization step in 3,711,407 the Soap-making stage of grease manufacture. INCORPORATENG LITHIUM SALCYLATE OR THE The aliphatic alcohol whose ester is used in this inven LKE ENTO A GREASE tion will preferably be one whose boiling point is not so Richard J. Plumstead, Mississauga, Ontario, Canada, as high as to prevent its being driven off during the reaction, signor to Esso Research and Engineering Company, 5 Linden, N.Y. unless it is desired to have all or a portion of the alcohol No Drawing. Filed Nov. 18, 1970, Ser. No. 90,878 remain in the composition. Reaction temperatures can Int, C. C10m 5/14 range from about 150 F. to about 550 F., more usually U.S. C. 252-4 6 Claims from about 180° F. to about 380 F. In the case of greases, residual alcohol in the grease may be objection 10 able in that it may exert excessive softening characteris ABSTRACT OF THE DISCLOSURE tics. On the other hand, some softening action may actu An alkali metal salt and particularly a lithium salt ally be desirable where it is necessary to have a high of Salicylic acid or related hydroxy benzoic acid is in thickener content and cost is a secondary consideration. corporated into a lubricating oil or grease as an antioxi Generally the esters will be of C1 to about Caliphatic 15 alcohols, but preferably they will be about C to Cs dant by Saponifying an aliphatic alcohol ester of the hy aliphatic alcohols, including methanol, ethanol, propanol, droxy benzoic acid and then removing the water and isopropanol, secondary butanol, 2-methylbutanol, 2,2-di alcohol formed in the reaction. -

Reintroduction of Lithium Into Recycled Electrode
(19) TZZ Z_T (11) EP 2 248 220 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: H01M 10/052 (2010.01) H01M 10/42 (2006.01) 04.11.2015 Bulletin 2015/45 H01M 10/54 (2006.01) H01M 4/485 (2010.01) H01M 4/505 (2010.01) H01M 4/525 (2010.01) (2010.01) (21) Application number: 09713108.0 H01M 4/58 (22) Date of filing: 20.02.2009 (86) International application number: PCT/US2009/034779 (87) International publication number: WO 2009/105713 (27.08.2009 Gazette 2009/35) (54) REINTRODUCTION OF LITHIUM INTO RECYCLED ELECTRODE MATERIALS FOR BATTERY WIEDEREINFÜGUNG VON LITHIUM IN VERWERTETE ELEKTRODENMATERIALIEN FÜR BATTERIEN RÉINTRODUCTION DE LITHIUM DANS DES MATÉRIAUX D’ÉLECTRODES RECYCLÉS POUR BATTERIES (84) Designated Contracting States: (56) References cited: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR EP-A1- 1 009 058 CN-A- 1 585 187 HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL JP-A- 11 054 159 US-A- 5 628 973 PT RO SE SI SK TR US-A- 5 679 477 US-A- 6 150 050 US-A1- 2003 180 604 US-A1- 2004 175 618 (30) Priority: 22.02.2008 US 30916 US-A1- 2005 003 276 US-A1- 2005 175 877 US-A1- 2005 244 704 US-A1- 2007 134 546 (43) Date of publication of application: US-A1- 2007 224 508 US-B1- 6 261 712 10.11.2010 Bulletin 2010/45 US-B2- 6 844 103 (73) Proprietor: Sloop, Steven E. -
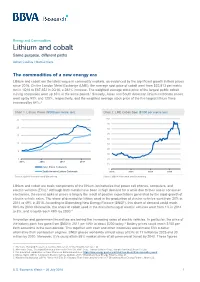
Lithium and Cobalt Same Purpose, Different Paths
Energy and Commodities Lithium and cobalt Same purpose, different paths Adrian Casillas / Marcial Nava The commodities of a new energy era Lithium and cobalt are the latest vogue in commodity markets, as evidenced by the significant growth in their prices since 2016. On the London Metal Exchange (LME), the average spot price of cobalt went from $22,813 per metric ton in 1Q16 to $87,352 in 2Q18, a 282% increase. The weighted average stock price of the largest public cobalt mining companies went up 85% in the same period.1 Similarly, Asian and South American lithium-carbonate prices went up by 93% and 120%, respectively, and the weighted average stock price of the five largest lithium firms increased by 84%.2 Chart 1. Lithium Prices ($’000 per metric ton) Chart 2. LME Cobalt Spot ($’000 per metric ton) 25 100 90 20 80 70 15 60 10 50 40 5 30 0 20 2015 2016 2017 2018 10 Asia Lithium Carbonate 0 South America Lithium Carbonate 2015 2016 2017 2018 Source: BBVA Research and Bloomberg Source: BBVA Research and Bloomberg Lithium and cobalt are basic components of the lithium-ion batteries that power cell phones, computers, and electric vehicles (EVs).3 Although both metals have been in high demand for a while due to their use in consumer electronics, the recent spike in prices is largely the result of positive expectations generated by the rapid growth of electric vehicle sales. The share of demand for lithium used in the production of electric vehicles went from 20% in 2014 to 49% in 2018.