Shock and Awe: a Dynamic Approach to Resuscitation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Effect of Passive Leg Raising Towards Hemodynamics on Patient with Hypovolemic Shock at the Emergency Ward of Dustira Cimahi Hospital
International Seminar on Global Health (ISGH) 2017 Stikes Jenderal Achmad Yani Cimahi The Effect of Passive Leg Raising towards Hemodynamics on Patient with Hypovolemic Shock at the Emergency Ward of Dustira Cimahi Hospital Evangeline Hutabarat Nursing Science Dept., Stikes Jenderal Achmad Yani Cimahi Email: [email protected] Abstract One of the conditions needing immediate response in the emergency ward is hypovolemic shock. The patient in shock requires close monitoring for clinical signs and hemodynamic status and intravascular status. It is due to circulatory assistance and medication provision on the patient is given based on the accuracy of the intravascular volume status of the patient. Fluid responsiveness can be considered as basis for considering administering fluid only or inotropic and vasopressor medication for the patient to maintain her circulatory homeostatic. Passive Leg Raising (PLR) may be a reversible method to evaluate fluid responsiveness. The study aims at evaluating the effect of PLR on hemodynamic. It employs the quasi-experimental design with the within subject repeated measurement design approach. 48 respondents took part as samples, with the consecutive sampling method implemented. The samples were then categorized into responsive and non-responsive groups based on the increase of pulse pressure amounting to or beyond 9% during PLR implementation. The data was tested using the dependent and independent t test to provide analysis for uni-variate and bivariate data. The results indicated that 34 respondents belonged to the responsive group while 14 respondents were categorized as non-responsive. There was a significant relationship between PLR and hemodynamic parameters of systolic blood pressure, diastolic blood pressure, mean arterial pressure, and pulse pressure (p>0.05). -

Fluid Resuscitation Therapy for Hemorrhagic Shock
CLINICAL CARE Fluid Resuscitation Therapy for Hemorrhagic Shock Joseph R. Spaniol vides a review of the 4 types of shock, the 4 classes of Amanda R. Knight, BA hemorrhagic shock, and the latest research on resuscita- tive fluid. The 4 types of shock are categorized into dis- Jessica L. Zebley, MS, RN tributive, obstructive, cardiogenic, and hemorrhagic Dawn Anderson, MS, RN shock. Hemorrhagic shock has been categorized into 4 Janet D. Pierce, DSN, ARNP, CCRN classes, and based on these classes, appropriate treatment can be planned. Crystalloids, colloids, dopamine, and blood products are all considered resuscitative fluid treat- ment options. Each individual case requires various resus- ■ ABSTRACT citative actions with different fluids. Healthcare Hemorrhagic shock is a severe life-threatening emergency professionals who are knowledgeable of the information affecting all organ systems of the body by depriving tissue in this review would be better prepared for patients who of sufficient oxygen and nutrients by decreasing cardiac are admitted with hemorrhagic shock, thus providing output. This article is a short review of the different types optimal care. of shock, followed by information specifically referring to hemorrhagic shock. The American College of Surgeons ■ DISTRIBUTIVE SHOCK categorized shock into 4 classes: (1) distributive; (2) Distributive shock is composed of 3 separate categories obstructive; (3) cardiogenic; and (4) hemorrhagic. based on their clinical outcome. Distributive shock can be Similarly, the classes of hemorrhagic shock are grouped categorized into (1) septic; (2) anaphylactic; and (3) neu- by signs and symptoms, amount of blood loss, and the rogenic shock. type of fluid replacement. This updated review is helpful to trauma nurses in understanding the various clinical Septic shock aspects of shock and the current recommendations for In accordance with the American College of Chest fluid resuscitation therapy following hemorrhagic shock. -

100% NON-INVASIVE FLUID MANAGEMENT Proper Fluid Management May Improve Clinical Outcomes, Potentially Saving Millions in Operating Costs1,2
100% NON-INVASIVE FLUID MANAGEMENT Proper Fluid Management May Improve Clinical Outcomes, Potentially Saving Millions in Operating Costs1,2 The Starling fluid management monitoring system provides a full hemodynamic profile within seconds. The effect of fluids can be monitored at any time and treatment modified accordingly, across the continuum of care: ED > ICU > OR > RRT In a retrospective, matched, single-center study of nearly 200 patients, researchers from the University of Kansas Health System evaluated stroke volume (SV) guided resuscitation in 100 ICU patients with severe sepsis and septic shock and found1: Initiation of Acute % ICU Length of Stay -2.89 DAYS Dialysis Therapy -13.25 Save an estimated Risk of Mechanical % $14,498 per Ventilation -51 treated patient3 $14K Starling - Over 80% of hospitalized patients receive IV fluids.4 Yet studies show that giving too little or too much fluid can lead to serious complications and contribute to rising healthcare costs.5,6 - Studies also show that only ~50% of hemodynamically unstable patients will respond to IV fluid by increasing cardiac output and perfusion. Assessing whether fluid may help or harm a patient is a critical step in optimizing treatment.7 - Using only blood pressure, urine output and heart rate to measure fluid responsiveness may provide limited and inconclusive information.7 TAKE THE GUESSWORK OUT OF YOUR FLUID ASSESSMENT WITH STARLING - Provides a dynamic assessment of fluid responsiveness — accurately, precisely and 100% non-invasively. - Supports individualized fluid therapy without requiring an invasive arterial or central line, potentially reducing the risk of hospital-acquired infections and other complications.8 - Independently validated vs. -

Passive Leg Raise Testing Effectively Reduces Fluid Administration In
Rameau et al. Ann. Intensive Care (2017) 7:2 DOI 10.1186/s13613-016-0225-6 RESEARCH Open Access Passive leg raise testing effectively reduces fluid administration in septic shock after correction of non‑compliance to test results Arjanne Rameau, Eldert de With and Evert Christiaan Boerma* Abstract Background: Fluid resuscitation is considered a cornerstone of shock treatment, but recent data have underlined the potential hazards of fluid overload. The passive leg raise (PLR) test has been introduced as one of many strategies to predict ‘fluid responsiveness.’ The use of PLR testing is applicable to a wide range of clinical situations and has the potential to reduce fluid administration, since PLR testing is based upon (reversible) autotransfusion. Despite these theoretical advantages, data on the net effect on fluid balance as a result of PLR testing remain scarce. Methods: We performed a prospective single-center multi-step interventional study in patients with septic shock to evaluate the effect of implementation of PLR testing on the fluid balance (FB) 48 hours after ICU admission. All patients were equipped with a PiCCO® device for pulse contour analysis to guide fluid administration. An increase in stroke volume (SV) 10% was considered a positive test result. ≥ Results: Before introduction of PLR testing, 21 patients were prospectively included in period 1 with a median FB of 4.8 [3.3–7.8]L. After an extensive training program, PLR testing was introduced and 20 patients were included in period 2. Median FB was 4.4 [3.3–7.5]L and did not differ from period 1 (p 0.72). -

The Use of Passive Leg Raise Test to Predict Hypotension in Patients Undergoing Urological Procedures Under Spinal Anesthesia
Abdullah MW, et al., J Anesth Clin Care 2017, 4: 021 DOI: 10.24966/ACC-8879/100021 HSOA Journal of Anesthesia and Clinical Care Clinical Perspective these outcomes were not related to an individual’s cardiac output The Use of Passive Leg Raise response to the PLR test before spinal anesthesia. Conclusion: The use of PLR test to predict the occurrence of hy- Test to Predict Hypotension in potension in patients undergoing urological procedures under spinal anesthesia was not useful. Patients Undergoing Urolog- Keywords: Passive leg raise; Spinal anesthesia; Spinal hypoten- ical Procedures under Spinal sion; Urologic procedures Introduction Anesthesia Transurethral surgeries are common surgical procedures that can Mai Wedad Abdullah, Mona Hossam EL Din Abdelhamid*, Nor- be performed under general or spinal anesthesia [1], spinal anesthesia han Abdel Aleim Ali and Atef Galal Abdel Moula has proved to be an excellent choice for such surgeries [2]. Hypoten- sion after spinal anesthesia remains a common and potentially seri- Department of Anesthesia and Pain Management, Faculty of Medicine, Cairo University, Egypt ous complication, despite the use of prophylactic ephedrine and fluid loading with crystalloids [3]. Hypotension during spinal anesthesia is due to sympathetic blockade which leads to vasodilatation with a reduction of systemic vascular resistance. Extensive block may lead to decreased venous return which is the main determinant of hypoten- Abstract sion during central neuraxial blockade [4]. Aim of study: A prospective observational study to examine wheth- Most patients scheduled for endoscopic urological surgery are el- er Passive Leg Raise Test (PLR) administered with non invasive car- derly and frequently suffered from cardiopulmonary, endocrine, or diac output monitoring by the electrical Cardiometry could be used other co morbidities [5]. -

Hemodynamic Profiles Related to Circulatory Shock in Cardiac Care Units
REVIEW ARTICLE Hemodynamic profiles related to circulatory shock in cardiac care units Perfiles hemodinámicos relacionados con el choque circulatorio en unidades de cuidados cardiacos Jesus A. Gonzalez-Hermosillo1, Ricardo Palma-Carbajal1*, Gustavo Rojas-Velasco2, Ricardo Cabrera-Jardines3, Luis M. Gonzalez-Galvan4, Daniel Manzur-Sandoval2, Gian M. Jiménez-Rodriguez5, and Willian A. Ortiz-Solis1 1Department of Cardiology; 2Intensive Cardiovascular Care Unit, Instituto Nacional de Cardiología Ignacio Chávez; 3Inernal Medicine, Hospital Ángeles del Pedregal; 4Posgraduate School of Naval Healthcare, Universidad Naval; 5Interventional Cardiology, Instituto Nacional de Cardiología Ignacio Chávez. Mexico City, Mexico Abstract One-third of the population in intensive care units is in a state of circulatory shock, whose rapid recognition and mechanism differentiation are of great importance. The clinical context and physical examination are of great value, but in complex situa- tions as in cardiac care units, it is mandatory the use of advanced hemodynamic monitorization devices, both to determine the main mechanism of shock, as to decide management and guide response to treatment, these devices include pulmonary flotation catheter as the gold standard, as well as more recent techniques including echocardiography and pulmonary ultra- sound, among others. This article emphasizes the different shock mechanisms observed in the cardiac care units, with a proposal for approach and treatment. Key words: Circulatory shock. Hemodynamic monitorization. -

Comparison of the Accuracy of Monitoring Devices in Prediction of Fluid Responsiveness in Severe Sepsis and Septic Shock
IRB# 15-21 COMPARISON OF THE ACCURACY OF MONITORING DEVICES IN PREDICTION OF FLUID RESPONSIVENESS IN SEVERE SEPSIS AND SEPTIC SHOCK PRINCIPAL INVESTIGATOR: JEFFREY C. FRIED, MD DATE OF PROTOCOL: 12/29/2016 VERSION 3 BACKGROUND One of the most challenging problems in critical care is determination of whether patients have adequate intravascular volume, and whether they would benefit from additional intravenous fluids. Giving too much fluid to patients with adequate intravascular volume could lead to fluid overload, congestive heart failure and subsequent respiratory failure. Not giving enough fluid to 1 IRB# 15-21 patients, risks leaving them under-resuscitated and worsening their shock state, leading to organ failures, such as kidney failure. Clinically, it is very difficult to determine this “intravascular volume status”, and predict which patients would benefit from more fluid. One of the ways we currently determine this, is to measure cardiac output and one of its main components, stroke volume (how much blood is pumped by the heart each beat) and see whether these increase in response to a passive leg raise (PLR) maneuver1. The maneuver is simple, quick, with essentially no risk. The maneuver involves measuring baseline cardiac output and stroke volume with the patient’s head up at 45 degrees and legs flat for 3 minutes. Subsequently, the head is placed flat and the legs are raised to 45 degrees for 3 minutes. If the cardiac output and stroke volume increase in response to this maneuver, there is a high probability that the patient will have a good response to giving intravenous fluid. Currently there are multiple FDA approved devices which can be used to measure cardiac output and stroke volume, ranging from very invasive (pulmonary artery catheter), to minimally invasive (requiring arterial catheter +/- central venous catheter- Flotrac, PICCO)3,4 to a newer, completely non- invasive device, called the NICOM (Cheetah). -

Passive Leg Raising for Fluid Responsiveness in Children: Is It Reliable? Javed Ismail1, Arun Bansal2
EDITORIAL Passive Leg Raising for Fluid Responsiveness in Children: Is it Reliable? Javed Ismail1, Arun Bansal2 Indian Journal of Critical Care Medicine (2020): 10.5005/jp-journals-10071-23430 Intravenous fluid administration is an integral part of management for critically ill children with impaired perfusion. Loss of more than 1Pediatric Intensive Care, NMC Royal Hospital, Abu Dhabi, United Arab 15–20% of intravascular volume impairs the preload which in turn Emirates results in decreased cardiac output, clinically manifesting with 2Department of Pediatrics, Advanced Pediatric Centre, Postgraduate signs of impaired perfusion or shock. In children, major causes Institute of Medical Education and Research, Chandigarh, India of shock, such as diarrhea, hemorrhage, sepsis, or anaphylaxis, Corresponding Author: Arun Bansal, Department of Pediatrics, result in either actual or relative intravascular hypovolemia (due to Advanced Pediatric Centre, Postgraduate Institute of Medical vasodilation). Rapid administration of fluid bolus could augment Education and Research, Chandigarh, India, Phone: +91 9815455002, preload to improve the cardiac output and, thus, can potentially e-mail: [email protected] reverse shock. However, studies demonstrate that only half of How to cite this article: Ismail J, Bansal A. Passive Leg Raising for the patients are preload responsive. In the other half, this fluid Fluid Responsiveness in Children: Is it Reliable? Indian J Crit Care Med accumulates and leaks into the interstitial space of almost all organs 2020;24(5):291–292. and can result in serious adverse effects. Fluid leak is exacerbated Source of support: Nil in inflammatory states such as sepsis; and about 95% of the given Conflict of interest: None fluid leaks out within 1 hour even among the fluid responders, thus limiting the beneficial effect.1 Hence, to identify which patient would respond to fluid bolus, tests of fluid responsiveness are recommended. -

Cardiac Arrest Due to Tension Pneumoperitoneum Caused by Esophagogastric Perforation and Pyloric Stenosis : a Case Report
Shinshu Med J, 67⑵:113~119, 2019 Cardiac Arrest Due to Tension Pneumoperitoneum Caused by Esophagogastric Perforation and Pyloric Stenosis : A Case Report 1 )* 1) 1) Hiroshi Miyama , Mayumi Okada , Hiroshi Takayama 1) 2) Hiroshi Imamura and Futoshi Muranaka 1) Department of Emergency and Critical Care Medicine, Shinshu University School of Medicine 2) Department of Surgery, Shinshu University School of Medicine Tension pneumothorax is one of the causes of sudden cardiac arrest with evidence of obstructive shock and subcutaneous emphysema. Emergency chest decompression is a treatment of choice in such a situation. Herein, we report a case of an out-of-hospital cardiac arrest due to tension pneumoperitoneum caused by esophagogas- tric perforation. A 40-year-old man with a history of duodenal ulcer and pyloric stenosis complained of sudden- onset abdominal pain and developed cardiac arrest during transportation to our hospital. He had jugular venous distention ; subcutaneous emphysema in the upper body trunk, arms, and neck ; and a markedly distended abdomen. Immediate needle-chest decompression was not effective, but after volume resuscitation, adrenaline administration, and abdominal decompression by nasogastric tube, spontaneous circulation was resumed. Radiological findings revealed tension pneumoperitoneum due to esophagogastric perforation. Emergency laparotomy was performed, and the perforation of the esophagogastric junction was detected. The patient was discharged from the hospital without any disability. Notably, in the treatment of a patient with cardiac arrest having subcutaneous emphysema, the cause of obstructive shock could exist not only in the chest, but also in the abdomen. Shinshu Med J 67 : 113―119, 2019 (Received for publication November 12, 2018 ; accepted in revised January 4, 2019) Key words : cardiac arrest, esophagogastric perforation, tension pneumoperitoneum decompression, successfully improved his circulation. -

Septic, Hypovolemic, Obstructive and Cardiogenic Killers
S.H.O.C.K Septic, Hypovolemic, Obstructive and Cardiogenic Killers Jason Ferguson, BPA, NRP Public Safety Programs Head, CVCC Amherst County DPS, Paramedic Centra One, Flight Paramedic Objectives • Define Shock • Review patho and basic components of life • Identify the types of shock • Identify treatments Shock Defined • “Rude unhinging of the machinery of life”- Samuel Gross, U.S. Trauma Surgeon, 1962 • “A momentary pause in the act of death”- John Warren, U.S. Surgeon, 1895 • Inadequate tissue perfusion Components of Life Blood Flow Right Lungs Heart Left Body Heart Patho Review • Preload • Afterload • Baroreceptors Perfusion Preservation Basic rules of shock management: • Maintain airway • Maintain oxygenation and ventilation • Control bleeding where possible • Maintain circulation • Adequate heart rate and intravascular volume ITLS Cases Case 1 • 11 month old female “not acting right” • Found in crib this am lethargic • Airway patent • Breathing is increased; LS clr • Circulation- weak distal pulses; pale and cool Case 1 • VS: RR 48, HR 140, O2 98%, Cap refill >2 secs • Foul smelling diapers x 1 day • “I must have changed her two dozen times yesterday” • Not eating or drinking much Case 1 • IV established after 4 attempts • Fluid bolus initiated • Transported to ED • Received 2 liters of fluid over next 24 hours Hypovolemic Shock Hemorrhage Diarrhea/Vomiting Hypovolemia Burns Peritonitis Shock Progression Compensated to decompensated • Initial rise in blood pressure due to shunting • Initial narrowing of pulse pressure • Diastolic raised -

Recognizing and Treating Shock in the Prehospital Setting
9/3/2020 Recognizing and Treating Shock in the Prehospital Setting 1 9/3/2020 Special Thanks to Our Sponsor 2 9/3/2020 Your Presenters Dr. Raymond L. Fowler, Dr. Melanie J. Lippmann, MD, FACEP, FAEMS MD FACEP James M. Atkins MD Distinguished Professor Associate Professor of Emergency Medicine of Emergency Medical Services, Brown University Chief of the Division of EMS Alpert Medical School Department of Emergency Medicine Attending Physician University of Texas Rhode Island Hospital and The Miriam Hospital Southwestern Medical Center Providence, RI Dallas, TX 3 9/3/2020 Scenario SHOCK INDEX?? Pulse ÷ Systolic 4 9/3/2020 What is Shock? Shock is a progressive state of cellular hypoperfusion in which insufficient oxygen is available to meet tissue demands It is key to understand that when shock occurs, the body is in distress. The shock response is mounted by the body to attempt to maintain systolic blood pressure and brain perfusion during times of physiologic distress. This shock response can accompany a broad spectrum of clinical conditions that stress the body, ranging from heart attacks, to major infections, to allergic reactions. 5 9/3/2020 Causes of Shock Shock may be caused when oxygen intake, absorption, or delivery fails, or when the cells are unable to take up and use the delivered oxygen to generate sufficient energy to carry out cellular functions. 6 9/3/2020 Causes of Shock Hypovolemic Shock Distributive Shock Inadequate circulating fluid leads A precipitous increase in vascular to a diminished cardiac output, capacity as blood vessels dilate and which results in an inadequate the capillaries leak fluid, translates into delivery of oxygen to the too little peripheral vascular resistance tissues and cells and a decrease in preload, which in turn reduces cardiac output 7 9/3/2020 Causes of Shock Cardiogenic Shock Obstructive Shock The heart is unable to circulate Obstruction to the forward flow of sufficient blood to meet the blood exists in the great vessels metabolic needs of the body. -
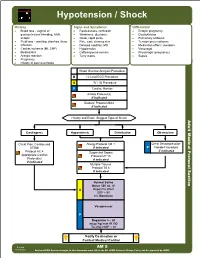
AM 05 Hypotension Shock Protocol Final 2017 Editable.Pdf
Hypotension / Shock History Signs and Symptoms Differential • Blood loss - vaginal or • Restlessness, confusion • Ectopic pregnancy gastrointestinal bleeding, AAA, • Weakness, dizziness • Dysrhythmias ectopic • Weak, rapid pulse • Pulmonary embolus • Fluid loss - vomiting, diarrhea, fever • Pale, cool, clammy skin • Tension pneumothorax • Infection • Delayed capillary refill • Medication effect / overdose • Cardiac ischemia (MI, CHF) • Hypotension • Vasovagal • Medications • Coffee-ground emesis • Physiologic (pregnancy) • Allergic reaction • Tarry stools • Sepsis • Pregnancy • History of poor oral intake Blood Glucose Analysis Procedure B 12 Lead ECG Procedure A IV / IO Procedure P Cardiac Monitor Airway Protocol(s) if indicated Diabetic Protocol AM 2 if indicated History and Exam Suggest Type of Shock Adult Medical Protocol Section Protocol Adult Medical Cardiogenic Hypovolemic Distributive Obstructive Chest Pain: Cardiac and Allergy Protocol AM 1 Chest Decompression- STEMI if indicated P Needle Procedure if indicated Protocol AC 4 Suspected Sepsis Appropriate Cardiac Protocol UP 15 Protocol(s) if indicated if indicated Multiple Trauma Protocol TB 6 if indicated A P Notify Destination or Contact Medical Control Revised AM 5 02/18/2019 Any local EMS System changes to this document must follow the NC OEMS Protocol Change Policy and be approved by OEMS Hypotension / Shock Adult Medical Protocol Section Protocol Adult Medical Pearls • Recommended Exam: Mental Status, Skin, Heart, Lungs, Abdomen, Back, Extremities, Neuro • Hypotension can be defined as a systolic blood pressure of less than 90. This is not always reliable and should be interpreted in context and patients typical BP if known. Shock may be present with a normal blood pressure initially. • Shock often is present with normal vital signs and may develop insidiously.