Transferrin Variants in Korea and Japan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ise-Shima National Park Lies on the Shima Peninsula, Located in the Center of Mie Prefecture in Western Japan
A scared landscape featuring the historical site of Ise Jingu Grand Shrine, as well as a picturesque coastal route and bountiful seascape 12 Ise-Shima Ise-Shima National Park lies on the Shima Peninsula, located in the center of Mie prefecture in Western Japan. Ise-Shima National Park includes the municipalities of Ise, Toba, Shima, and Minami-Ise, occupying a vast area of National Park nearly 60,000 hectares. The park is broadly divided into two areas: the inland area which is home to Ise Jingu and its surrounding forests; and the coastal area which is characterized by an archipelagic landscape with intricate ria coasts. The interaction between people and nature is very profound in the region, and the essence of this relationship can be observed in the ancient tradition of ama, female diver, fishing, among other traditional cultural practices that have developed around Ise Jingu over the millennia. The wisdom to enjoy the bounties of nature in a sustainable manner is figured prominently in Ise Jinguʼs Shikinen Sengu, a ritual held once every 20 years whereby the shrine is rebuilt in its entirety. The region has a long history of providing seafood to the Imperial Court and Ise Jingu, and the Manyoshu (Japanʼs oldest poetry compilation) refers to the region as Miketsu Kuni or the "land of divine off erings". Thus, the region boasts a rich marine environment home to diverse aquatic life, including Japanese spiny lobster, abalones, turban shells, oysters, tunas, red sea breams, and various types of seaweed. The Kuroshio sea current has blessed the region with a warm climate, which has led to the development of Ise Jinguʼs lush forest and dense evergreen forests that cling to the foothills of the mountainous regions. -

Notice of Decision of Issuance Conditions for Sustainability Bond
(Translation) February 19, 2021 Dear Sirs and Madams Name of the Company: NH Foods Ltd. Representative: Yoshihide Hata President and Representative Director (Code No. 2282, First Section of the Tokyo Stock Exchange) Person to contact: Tomoya Matsuda Public & Investor Relations Department (TEL: 06-7525-3031) Notice of Decision of Issuance Conditions for Sustainability Bond It is hereby notified that NH Foods Ltd. (the “Company”), has determined today that the sustainability bonds (14th Unsecured Corporate Bond) (“NH Foods Sustainability Bond”) will be issued under the following conditions. Description I. Purpose and Background of Issuance The Company has included “Pursue sustainability” as one of the business policies for the “Medium- Term Management Plan 2020” which is a business plan covering the three years from the year starting April 1, 2018. Furthermore, in January 2016, the Company identified the “Five CSR Material Issues” and is working to resolve environmental and social issues through our businesses. The Company will contribute to the achievement of sustainable environment and society by applying the funds raised through this issuance to expenditures related to construction of the new baseball stadium (ES CON FIELD HOKKAIDO) which received a five-star rating under the DBJ Green Building Certification system. II. Overview of NH Foods Sustainability Bond NH Foods Ltd. 14th Unsecured Corporate Bond (limited inter- (1) Name of bond bond pari passu rider) (Sustainability Bond) (2) Total amount of issue 10 billion yen (3) Interest rate 0.300% per annum (4) Issue date February 26, 2021 (5) Maturity date (Term) February 20, 2031 (10 years) (6) Interest payment date February 20 and August 20 of each year - 1 - A + (Japan Credit Rating Agency, Ltd.) (7) Bond rating A (Rating and Investment Information, Inc.) (8) Third-party evaluation Compatible with the “Sustainability Bond Guidelines 2018” and on sustainability finance “Green Bond Guidelines 2020”, obtained the second opinion from framework Sustainalytics which is an independent evaluation organization. -

The 1964 Tokyo Games Reconsidered
Volume 18 | Issue 5 | Number 11 | Article ID 5369 | Mar 01, 2020 The Asia-Pacific Journal | Japan Focus Symbolic Transformation: The 1964 Tokyo Games Reconsidered Christian Tagsold Abstract: The 1964 Tokyo Olympics facilitated that began with the torch relay in Okinawa, Japan’s symbolic rebirth in the wake of World which remained under US administration until War II. Infrastructural projects like the1972, and ended with a ‘victim’ of the Shinkansen were intentionally blended with Hiroshima atomic bombing lighting the remnants of ultra-nationalism into a new type Olympic cauldron in Tokyo. of post-war patriotism. The games sanctioned Japan’s redemption and reinforced a sense of After the Tokyo Olympics ended, Ishihara national purpose and collective identity while Shintarō wrote, “It is a bit frivolous to dwell on providing a stage for Emperor Hirohito’s the Olympics regarding the fate of the state rehabilitation. In subtle ways the Olympics and its people, since the relationship between created an opportunity to rebrand Japan as both is substantially weak. But since we have modern and cutting edge while alsothis opportunity anyway, why not start by symbolically embracing a history and traditions slowly reconsidering ourselves, and our present that had been implicated and discredited by situation?” (Ishihara 2014 [1964]: 303) The wartime depredations. games taught Ishihara to respect and appreciate the beauty of athletes’ relentless struggles. He admired their discipline and willingness to sacrifice, virtues he believed Discussion of the 1964 Summer Olympics tends were lacking among his postwar Japanese to focus on the massive infrastructure projects compatriots. and Japan’s pell-mell postwar modernization, but it is also important to examine the symbolic In 1999, Ishihara Shintarō was elected implications of the games. -

The Debate on the Introduction of a Regional System in Japan
Up-to-date Documents on Local Autonomy in Japan No.3 The Debate on the Introduction of a Regional System in Japan Kiyotaka YOKOMICHI Professor National Graduate Institute for Policy Studies (GRIPS) Council of Local Authorities for International Relations (CLAIR) Institute for Comparative Studies in Local Governance (COSLOG) National Graduate Institute for Policy Studies (GRIPS) Except where permitted by the Copyright Law for “personal use” or “quotation” purposes, no part of this booklet may be reproduced in any form or by any means without the permission. Any quotation from this booklet requires indication of the source. Contact: Council of Local Authorities for International Relations (CLAIR) (International Information Division) Shin Kasumigaseki Building 19F, 3-3-2 Kasumigaseki, Chiyoda-ku, Tokyo 100-0013 Japan TEL: 03 - 3591 - 5482 FAX: 03 - 3591 - 5346 Email: [email protected] Institute for Comparative Studies in Local Governance (COSLOG) National Graduate Institute for Policy Studies(GRIPS) 7-22-1 Roppongi, Minato-ku, Tokyo 106-8677 Japan TEL: 03 - 6439 - 6333 FAX: 03 - 6439 - 6010 Email: [email protected] Foreword The Council of Local Authorities for International Relations (CLAIR) and the National Graduate Institute for Policy Studies (GRIPS) have been working since 2005 on a “Project on the overseas dissemination of information on the local governance system of Japan and its operation”. On the basis of the recognition that the dissemination to overseas countries of information on the Japanese local governance system and its operation was insufficient, the objective of this project was defined as the pursuit of comparative studies on local governance by means of compiling in foreign languages materials on the Japanese local governance system and its implementation as well as by accumulating literature and reference materials on local governance in Japan and foreign countries. -

Vol.22 August 2001
vol.22 August 2001 ~Think Together about the Capital Functions Relocation of Japan~� Trends in the Diet ■ House of Representatives� The Special Committee for the Relocation of the Diet and Other Organizations (Chaired by Mr. Hide- toshi Nagai) dispatched its members to the "Gifu-Aichi Region" (on July 2), "Tochigi-Fukushima Region" (on July 3) and "Mie-Kio Region" (on July 31).� In the "Gifu-Aichi Region," the members were given general explanations by Governor Taku Kajiwara of Gifu Prefecture and Governor Masaaki Kanda of Aichi Prefecture on the spot and conducted field sur- vey of major locations in the "Aichi-Gifu Region," including aerial inspection using a helicopter.� In the "Tochigi-Fukushima Region," the members were given general explanations by Governor Akio Fukuda of Tochigi Prefecture and Governor Eisaku Sato of Fukushima Prefecture on the spot and con- ducted field survey of areas of interest, such as the Nasuno-ga-hara in Tochigi Prefecture and surround- ing areas of the Fukushima Airport in Fukushima Prefecture.� In the "Mie-Kio Region," the members were given general explanations by Governor Masayasu Kita- gawa of Mie Prefecture, Governor Yoshitsugu Kunimatsu of Shiga Prefecture, Governor Teiichi Aramaki of Kyoto Prefecture, and Governor Yoshiya Kakimoto of Nara Prefecture, and conducted field survey of areas of interest, such as the Ayama Town Observation Facility. The Special Committee for the Relocation of the Diet and� Other Organizations of the House of Representatives Has Inaugurated Its Homepage The Special Committee for the Relocation of the Diet and Other Organizations of the House of Representatives is opening its homepage to solicit citizens' opinions about the capital functions relocation. -

Osaka Gas to Start Service to Purchase Surplus Electricity Generated by Photovoltaic Systems (For Customers Whose Feed-In Tariff
Osaka Gas to Start Service to Purchase Surplus Electricity Generated by Photovoltaic Systems (for customers whose feed-in tariff period for renewable energy will end) May 15, 2019 Osaka Gas Co., Ltd. Osaka Gas Co., Ltd. (President: Takehiro Honjo) will launch a service to purchase surplus electricity generated by photovoltaic systems. The service is available for customers whose purchase period (10 years) will end under the renewable energy feed-in-tariff (FIT) system. Because the FIT purchase period is ten years, customers whose FIT purchase period expires will be able to choose purchasers freely*1 from November 2019. Osaka Gas will offer the Purchase Plan for electricity, which is available for any customer, and the Electricity Set Purchase Plan, which offers an advantage to customers who use Osaka Gas’s electricity. Osaka Gas will also offer the Style Plan E option, in which the purchase unit price increases further in combination with Style Plan E,*2 an environmentally friendly electricity plan. Osaka Gas has been promoting the spread of ENE-FARM, photovoltaic power generation systems, storage batteries, and other solutions to help achieve a low carbon society. The company has also been working to upgrade the electricity charge plan to meet customers’ diverse values and lifestyles. The company also has a track record*3 in selling electricity throughout the Kinki region. We remain committed to offering safe and secure high-quality services for reliable use of energy and other services that contribute to customers’ daily lives and business operations, in order to become an energy marketer that continues to be chosen by customers in the new era. -

Japanese Geography Quiz What Prefecture Is Located in the Northernmost Part in Japan?
Japanese Geography Quiz What prefecture is located in the northernmost part in Japan? ① Hokkaido ② Aomori ③ Akita ④ Ishikawa What prefecture is located in the northernmost part in Japan? ① Hokkaido ② Aomori ③ Akita ④ Ishikawa What prefecture is located in the westernmost part in Japan? ① Okinawa ② Nagasaki ③ Osaka ④ Kagoshima What prefecture is located in the westernmost part in Japan? ① Okinawa ② Nagasaki ③ Osaka ④ Kagoshima What is the name of the island located in the southernmost part of Japan? ① Yaku Island ② Marcus Island ③ Okinawa Island ④ Okinotori Islands What is the name of the island located in the southernmost part of Japan? ① Yaku Island ② Marcus Island ③ Okinawa Island ④ Okinotori Islands What is the name of the island located in the easternmost part of Japan? ① Father Island ② Okinotori Islands ③ Marcus Island ④ Mother Island What is the name of the island located in the easternmost part of Japan? ① Father Island ② Okinotori Islands ③ Marcus Island ④ Mother Island What mountain is the second highest in Japan following Mt. Fuji? ① Yatsugatake Mountains ② Mt. Hotaka ③ Monte Yari ④ Mt. Kita (Shirane) What mountain is the second highest in Japan following Mt. Fuji? ① Yatsugatake Mountains ② Mt. Hotaka ③ Monte Yari ④ Mt. Kita (Shirane) How high is Mt.Fuji, the highest mountain in Japan? ① 1,776 meters ② 2,776 meters ③ 3,776 meters ④ 4,776 meters How high is Mt.Fuji, the highest mountain in Japan? ① 1,776 meters ② 2,776 meters ③ 3,776 meters ④ 4,776 meters What is the longest river in Japan? ① Shinano River ② Tone River ③ Ishikari -

List of UNITI-1 Investigators in Japan List of UNITI-2 Investigators in Japan List of IM-UNITI Investigators in Japan Dose Adjus
See “Efficacy and safety of ustekinumab in Japanese patients with moderately to severely active Crohn’s disease: a subpopu- lation analysis of phase 3 induction and maintenance studies” on page 475-486. Supplementary Information List of UNITI-1 investigators in Japan A Ando, Shiga University of Medical Science Hospital, Otsu, Shiga; T Ashida, Sapporo Higashi Tokushukai Hospital, Sapporo, Hokkaido; H Hanai, Hamamatsu South Hospital, Minamiku, Hamamatsu; T Ishida, Oita Red Cross Hospital, Oita City, Oita; H Ito, Kinshu-kai Infusion Clinic, Osaka City, Osaka; T Matsumoto, Kyushu University Hospital, Fukuoka city, Fukuoka; S Motoya, Hokkaido P.W.F.A.C. Sapporo-Kosei General Hospital, Sapporo-shi, Hokkaido; S Nakamura, Hyogo College of Medicine, Nishi- nomiya, Hyogo; Y Sameshima, Sameshima Hospital, Kagoshima-city, Kagoshima; Y Suzuki, Toho University, Sakura Medical Center, Sakura-shi, Chiba; K Watanabe, Osaka City University Hospital, Osaka City, Osaka; H Yamagami, Osaka City University Hospital, Osaka City, Osaka; T Yamamoto, Yokkaichi Social Insurance Hospital, Department of Surgery, Yokkaichi, Mie; K Yao, Fukuoka University Chikushi Hospital, Chikushi, Fukuoka. List of UNITI-2 Investigators in Japan T Ashida, Sapporo Higashi Tokushukai Hospital, Sapporo, Hokkaido; H Hanai, Hamamatsu South Hospital, Minamiku, Ham- amatsu; N Horiki, Mie University Hospital, Mie Prefecture, Tsu; H Iijima, Osaka University Hospital Department of Medicine, Gastroenterology and Hepatology, Suita; T Ishida, Oita Red Cross Hospital, Oita City, Oita; H Ito, Kinshu-kai Infusion Clinic, Osaka City, Osaka; M Iwabuchi, National Hospital Organization Sendai Medical Center, Miyagi Prefecture, Sendai; T Kanai, Keio University Hospital, Shinjuku-ku, Tokyo; R Kunisaki, Yokohama City University Medical Center, Kanagawa, Minami-ku, Yo- kohama; A Maemoto, Sapporo Higashi Tokushukai Hospital, Sapporo, Hokkaido; K Matsuoka, Keio University Hospital, Shin- juku-ku, Tokyo; S Motoya, Hokkaido P.W.F.A.C. -

Mie's Opportunities Developed by Adopting ICT Aggressively
Mie’s Opportunities Developed by Adopting ICT Aggressively Diffusion of Cashless Payments in Mie Mie’s cashless payment ratio Our target penetration ratio of cashless payments in Mie Toward a prefecture leading in cashless payments 1st Tokyo (1) Inbound tourists Credit card 2nd Kagawa (2) Large shopping 50% centers 3rd Chiba Debit card 40% 2025 Gov’t 4th Mie 19% 19.15 % E-Money Target 40% (Prepaid card) Mie Pref. is more receptive to cashless payments * Based on Commercial Statistics announced by the Government (2014) 2014 2023 2025 Smartphone settlement (QR code, etc.) * Announced by the Ordinary Committee Meeting of Strategy, Planning, Employment and economy of Mie Prefecture Congress Activities to diffuse cashless payments in Oct. 2019 Hyakugo Bank Cashless Large Individual and small business Cashless payment of automobile taxes companies SMEs owners Seminar based on our Bar Code Pay for retailers and restaurant Large owners sales Encourage a wide range of SMEs, individuals, and April 2020 Already small business owners Full-scale introduction by Mie pref. adopted Market with potential users of cashless payments Accelerate use of Small sales Bar Code Pay OrigamiPay Promotion Seminar Ranked low in sales 4 Improving Tourism Brand Power Using ICT Number of tourists Tourist spending in Mie pref. Mie pref. total (based on (1,000 (100 million yen) nationwide standard) people) 45,000 6,000 42,605 Marked record high sales in 5,338 Ise Shrine 42,195 tourism-related market 5,273 Shikinen Sengu 41,892 4,897 40,799 3,968 40,000 4,000 3,840 37,868 -
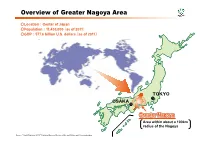
Overview of Greater Nagoya Area
Overview of Greater Nagoya Area 〇Location: Center of Japan 〇Population:11,430,000( as of 2011) 〇GDP:577.6 billion U.S. dollars( as of 2011) TOKYO OSAKA Greater Nagoya Area within about a 100km radius of the Nagoya Source: "World Statistics 2013," Statistics Bureau, Ministry of Internal Affairs and Communications International Comparisons of Greater Nagoya 〇GDP of Greater Nagoya exceeds that of Belgium( USD). 〇Per capita GDP of Greater Nagoya is as big as that of the U.S. ★GDP international comparison( 2011)( USD) ★Per capita GDP international comparison(2011)( USD) (Unit: billion U.S. dollars) (Unit: U.S. dollars) Greater Nagoya 577.6 Greater Nagoya 50,500 Japan 5,904.7 Japan 46,192 United States 14,991.3 United States 47,882 China 7,203.8 China 5,439 Germany 3,604.1 Germany 43,865 France 2,775.5 France 42,642 India 1,897.6 India 1,528 South Korea 1,116.2 South Korea 23,067 Netherlands 836.8 Netherlands 50,215 Indonesia 846.8 Indonesia 3,495 Belgium 514.1 Belgium 47,807 ★Population: Comparison(2011) (Thousands) Greater Nagoya 11,437 Japan 127,799 United States 311,592 China 1,344,100 Germany 81,798 France 63,294 Source: "World Statistics 2013," Statistics Bureau, Ministry of Internal Affairs and Communications India 1,192,503 Note 1. The values of the Greater Nagoya are those of the Tokai region. South Korea 50,111 2. The Greater Nagoya calculate the values of FY2011 while other countries use the values of Netherlands 16,693 2011. 3. -

MIE PREFECTURE Latest Update: August 2013
www.EUbusinessinJapan.eu MIE PREFECTURE Latest update: August 2013 Prefecture’s Flag Main City: Tsu Population: 1,830,000 people, ranking 22/47 prefecture (2013) [1] Area: 5,777 km2 [2] Geographical / Landscape description Mie Prefecture forms the eastern part of the Kii Peninsula, and borders on Aichi, Gifu, Shiga, Kyoto, Nara, and Wakayama. It is well located between Nagoya, Osaka and Kyoto making the three cities in easy reach. It has a varied landscape that includes mountains, plains and coastline. Nearly one third of the total area of the prefecture is designated as nature reserves and parks, one of the highest rates in Japan. [2] Climate The climate is warm with an average annual temperature of 14 to 15 °C. The north features snow falls, while the southern region is known as one of the rainiest areas in Japan. [2] Time zone GMT +7 in summer (+8 in winter) International dialling code: 0081 Recent history, culture The prefecture is home of the Ise Jingu shrine. It is the supreme shrine where Amaterasu Omikami, the ancestral goddess of the Imperial Family and the overall patron goddess of Japan, worshipped nearly 2000 years ago. One of the peculiarities of Ise Jingu is the renewal ceremony that takes place every 20 years during which the old shrine is demolished and rebuilt nearby using the same construction techniques used in past centuries. [3] Economic overview Mie Prefecture contains one of Japan’s foremost petrochemical complexes and maintains a strong concentration of advanced material manufacturing technology companies. In addition, the prefecture boasts well developed automotive, semiconductor, liquid crystal, medical and health and welfare industries. -

Mie Prefecture Ise-Shima
Mie & Ise-Shima Mie Prefecture Mie Prefecture, where the Ise-Shima area is located, is positioned almost in the center of the Tokai region on the Japanese archipelago, facing the Pacific Ocean. Osaka โอซาก้า Tokyo Long and thin from north to south, โตเกีย the prefecture is about Nagoyaว 80 km from east to west Mie นาโกย่า มิเอะ and 170 km from north to south. Hokusei Area Ise-Shima เขตโฮกุเซ Chunansei Area Iga Area The Ise-Shima area is located in the southeastern part of Mie เขตจูนันเซ เขตอิกะ Prefecture. The area is dotted with numerous shrines and temples, including Ise Jingu, which is often called the “hometown of the hearts of the Japanese” and is a charming area steeped in history and tradition. Higashikishu Area เขตฮงิ าชคิ ชิ ู The rich, beautiful natural environment that is a part of Ise-Shima Area Ise-Shima National Park has a delicate and graceful landscape, เขตอเิ สะชมิ ะ and the wealth of seafood nurtured on the jagged rias coastline has brought countless blessings to people since ancient times. Ise Toba Shima Ise Grand Shrine Meotoiwa Rocks Toba Aquarium Mikimoto Pearl Island Yokoyama Observatory Kashikojima Espana Cruise Oharaimachi Okage Yokocho Ama Hut Pearls Ama Hut Shima Nature School Aka Fuku Mochi Ise Udon Toba oyster Horned Turban Japanese Spiny Lobster Tekone Sushi Ise's climate is relatively temperate. It is a city Toba is renowned for its temperate climate and The entire city area has been designated as part of blessed with unique local assets. Ise has a wealth the picturesque, island-studded Ise Bay.