(IL-7) Selectively Promotes Mouse and Human IL-17–Producing Γδ Cells
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse
Welcome to More Choice CD Marker Handbook For more information, please visit: Human bdbiosciences.com/eu/go/humancdmarkers Mouse bdbiosciences.com/eu/go/mousecdmarkers Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse CD3 CD3 CD (cluster of differentiation) molecules are cell surface markers T Cell CD4 CD4 useful for the identification and characterization of leukocytes. The CD CD8 CD8 nomenclature was developed and is maintained through the HLDA (Human Leukocyte Differentiation Antigens) workshop started in 1982. CD45R/B220 CD19 CD19 The goal is to provide standardization of monoclonal antibodies to B Cell CD20 CD22 (B cell activation marker) human antigens across laboratories. To characterize or “workshop” the antibodies, multiple laboratories carry out blind analyses of antibodies. These results independently validate antibody specificity. CD11c CD11c Dendritic Cell CD123 CD123 While the CD nomenclature has been developed for use with human antigens, it is applied to corresponding mouse antigens as well as antigens from other species. However, the mouse and other species NK Cell CD56 CD335 (NKp46) antibodies are not tested by HLDA. Human CD markers were reviewed by the HLDA. New CD markers Stem Cell/ CD34 CD34 were established at the HLDA9 meeting held in Barcelona in 2010. For Precursor hematopoetic stem cell only hematopoetic stem cell only additional information and CD markers please visit www.hcdm.org. Macrophage/ CD14 CD11b/ Mac-1 Monocyte CD33 Ly-71 (F4/80) CD66b Granulocyte CD66b Gr-1/Ly6G Ly6C CD41 CD41 CD61 (Integrin b3) CD61 Platelet CD9 CD62 CD62P (activated platelets) CD235a CD235a Erythrocyte Ter-119 CD146 MECA-32 CD106 CD146 Endothelial Cell CD31 CD62E (activated endothelial cells) Epithelial Cell CD236 CD326 (EPCAM1) For Research Use Only. -

Type I Interferons and the Development of Impaired Vascular Function and Repair in Human and Murine Lupus
Type I Interferons and the Development of Impaired Vascular Function and Repair in Human and Murine Lupus by Seth G Thacker A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Immunology) in The University of Michigan 2011 Doctoral Committee: Associate Professor Mariana J. Kaplan, Chair Professor David A. Fox Professor Alisa E. Koch Professor Matthias Kretzler Professor Nicholas W. Lukacs Associate Professor Daniel T. Eitzman © Seth G Thacker 2011 Sharon, this work is dedicated to you. This achievement is as much yours as it is mine. Your support through all six years of this Ph.D. process has been incredible. You put up with my countless miscalculations on when I would finish experiments, and still managed to make me and our kids feel loved and special. Without you this would have no meaning. Sharon, you are the safe harbor in my life. ii Acknowledgments I have been exceptionally fortunate in my time here at the University of Michigan. I have been able to interact with so many supportive people over the years. I would like to express my thanks and admiration for my mentor. Mariana has taught me so much about writing, experimental design and being a successful scientist in general. I could never have made it here without her help. I would also like to thank Mike Denny. He had a hand in the beginning of all of my projects in one way or another, and was always quick and eager to help in whatever way he could. He really made my first year in the lab successful. -

Anti-CD40 Antibody KPL-404 Inhibits T Cell
Marken et al. Arthritis Research & Therapy (2021) 23:5 https://doi.org/10.1186/s13075-020-02372-z RESEARCH ARTICLE Open Access Anti-CD40 antibody KPL-404 inhibits T cell- mediated activation of B cells from healthy donors and autoimmune patients John Marken1, Sujatha Muralidharan2* and Natalia V. Giltiay1* Abstract Background: CD40-CD40L is a key co-stimulatory pathway for B cell activation. As such, its blockade can inhibit pathogenic B cell responses in autoimmune diseases, such as Sjogren’s syndrome (SjS) and systemic lupus erythematosus (SLE). In this study, we examined the in vitro effects of KPL-404, a humanized anti-CD40 monoclonal antibody (Ab), on primary human B cells derived from either healthy donors (HD) or autoimmune patients and compared them to the effects of G28-5, a partially antagonistic anti-CD40 antibody. Methods: PBMCs from HD or SjS and SLE patients were cultured in high-density cell cultures in the presence of IgG4 isotype control or anti-CD40 Abs KPL-404 or G28-5. Cells were stimulated with anti-CD3/CD28 cross-linking reagent ImmunoCult (IC) to induce CD40L-CD40-mediated B cell responses. B cell proliferation and activation, measured by dilution of proliferation tracker dye and the upregulation of CD69 and CD86, respectively, were assessed by flow cytometry. Anti-CD40 Ab cell-internalization was examined by imaging flow cytometry. Cytokine release in the PBMC cultures was quantified by bead-based multiplex assay. Results: KPL-404 binds to CD40 expressed on different subsets of B cells without inducing cell depletion, or B cell proliferation and activation in in vitro culture. -

Maturation and Is Inhibited by TLR2 Signaling Through TLR4 Promotes
Role of TLR in B Cell Development: Signaling through TLR4 Promotes B Cell Maturation and Is Inhibited by TLR2 This information is current as Elize A. Hayashi, Shizuo Akira and Alberto Nobrega of September 28, 2021. J Immunol 2005; 174:6639-6647; ; doi: 10.4049/jimmunol.174.11.6639 http://www.jimmunol.org/content/174/11/6639 Downloaded from References This article cites 45 articles, 26 of which you can access for free at: http://www.jimmunol.org/content/174/11/6639.full#ref-list-1 Why The JI? Submit online. http://www.jimmunol.org/ • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average by guest on September 28, 2021 Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2005 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Role of TLR in B Cell Development: Signaling through TLR4 Promotes B Cell Maturation and Is Inhibited by TLR21 Elize A. Hayashi,* Shizuo Akira,† and Alberto Nobrega2* The role of TLR4 in mature B cell activation is well characterized. -

Association of CD69 and CD 25 Activation Markers on CD4 And
arm Ph ac f ov l o i a g n il r a n u c o e J Teixeira et al., J Pharmacovigilance 2013, 1:3 Journal of Pharmacovigilance DOI: 10.4172/2329-6887.1000111 ISSN: 2329-6887 CaseResearch Report Article OpenOpen Access Access Association of CD69 and CD 25 Activation Markers on CD4 and CD8 Cells with Skin Tests in Drug Allergy Teixeira FM1, Vasconcelos LMF2, Araújo TS3, Genre J4, Almeida TLP5, Magalhães HIF3, Câmara LMC6 and Nagao-Dias AT3* 1Posgraduation Program of Biotechnology (RENORBIO), Federal University of Ceará (UFC), Brazil 2Posgraduation Program of Pharmaceutical Sciences, UFC, Brazil 3Department of Clinical Analysis and Toxicology, Faculty of Pharmacy, UFC, Brazil 4Department of Clinical Analysis and Toxicology, Faculty of Pharmacy, Federal University of Rio Grande do Norte, Brazil 5Department of Dermatology, Hospital Universitário Walter Cantídio, UFC, Brazil 6Department of Pathology, Faculty of Medicine, UFC, Brazil Abstract Background: Diagnosis of drug allergy is difficult because few methods have been validated in the literature. In the last few years, identification of T cell activation markers to assess drug allergy has been the focus of several studies. Objective: The aim of the present work was to search for CD25 and CD69 markers on T CD4+ and T CD8+ cells in drug allergy. Methods: Fourteen patients with drug hypersensitivity were enrolled in this investigation. Some patients had at least one adverse reaction to one or more suspected drugs, therefore, a total of 16 reactions and 10 drugs were investigated. Prick or patch tests were done according with the time of onset and type of the clinical manifestations. -

BD Multicolor Antibody Reagents Catalog BD Continues to Provide More Choices for Multicolor Flow Cytometry Applications by Expanding Our Portfolio and Color Options
Welcome to More Choice BD Multicolor Antibody Choose from our extensive portfolio of high-quality fluorescent-conjugated reagents Reagents Catalog to build your multicolor flow cytometry panels. 10th Edition Human Mouse Non-Human Primate Welcome to More Choice BD Multicolor Antibody Reagents Catalog BD continues to provide more choices for multicolor flow cytometry applications by expanding our portfolio and color options. Check out the newly released BD Horizon™ Brilliant Violet™ reagents: Human • BD Horizon™ BV421: Offers PE-level brightness for the violet laser, making it optimal for dim markers. It can be detected in the BD Horizon V450 filter set (450/50 nm). Mouse • BD Horizon™ BV510: Adds another bright choice for the violet laser. It can be detected in Non-Human Primate the BD Horizon V500 filter set (525/20 nm). • BD Horizon™ BV605: Provides a very bright option for the third violet channel. • BD Horizon™ BV711: Provides a fourth dye for the violet laser expanding the options for multicolor flow. The brightness of this dye makes it ideal for dim markers. • Look for newly released products as we continue our commitment to provide more choices for multicolor assays. More Sizes BD now offers a wide variety of antibody conjugates in small and large vial sizes. Small sizes (25 test or 25 µg) are now available for hundreds of specificities. • Use the small-size formats to determine feasibility of pilot experiments. • Design and optimize multicolor panels with our small sizes. Larger sizes (250 test or 500 test) are now available for many of the common markers. • Use this convenient package size for routine assays or large studies. -

IL-7) and IL-7 Splice Variants Affect Differentiation of Human Neural Progenitor Cells
Genes and Immunity (2010) 11, 11–20 & 2010 Macmillan Publishers Limited All rights reserved 1466-4879/10 $32.00 www.nature.com/gene ORIGINAL ARTICLE Interleukin-7 (IL-7) and IL-7 splice variants affect differentiation of human neural progenitor cells M Moors1,4, NK Vudattu2,, J Abel1, U Kra¨mer1, L Rane2, N Ulfig3, S Ceccatelli4, V Seyfert-Margolies5, E Fritsche1 and MJ Maeurer2 1Group of Toxicology, Group of Epidemiology, Institut fu¨r Umweltmedizinische Forschung, Du¨sseldorf, Germany; 2Microbiology, Tumor and Cell Biology and Smittskyddsinstitutet, Stockholm, Sweden; 3Department of Anatomy, University of Rostock, Rostock, Germany; 4Division of Neurotoxicology, Department of Neuroscience, Karolinska Institutet, Sweden and 5Department of Medicine, University of California, San Francisco, CA, USA Alternative splicing of pre-mRNA increases proteomic diversity, a crucial mechanism in defining tissue identity. We demonstrate differentially spliced interleukin (IL)-7 in distinct anatomic areas in the adult, in developing human brains and in normal human neuronal progenitor (NHNP) cells. IL-7c (c, the canonical form spanning all six exons) or its variants IL-7d5, d4 or d4/5 were cloned and expressed as recombinant proteins. IL-7 and splice variants were able to shift the differentiation of NHNP cells as compared with the diluent control (Po0.01) defined by anti-b (III)-tubulin and glial fibrillary acidic protein expression, with different degrees (IL-7c4d4/54IL-7d5); IL-7d4 exhibited a significantly weaker potency. Differentiation was confirmed by transcriptome analysis of IL-7c-stimulated neural NHNP cells, resulting in 58 differentially expressed genes; some of these are involved in neural differentiation, for example, the developmentally regulated transcription factor kru¨ppel-like factor 12, musashi 2, a translational regulator of cell fate or the sonic hedgehog receptor patch 1. -
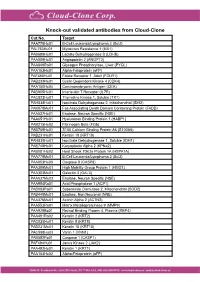
Knock-Out Validated Antibodies from Cloud-Clone Cat.No
Knock-out validated antibodies from Cloud-Clone Cat.No. Target PAA778Hu01 B-Cell Leukemia/Lymphoma 2 (Bcl2) PAL763Hu01 Myxovirus Resistance 1 (MX1) PAB698Hu01 Lactate Dehydrogenase B (LDHB) PAA009Hu01 Angiopoietin 2 (ANGPT2) PAA849Ra01 Glycogen Phosphorylase, Liver (PYGL) PAA153Hu01 Alpha-Fetoprotein (aFP) PAF460Hu01 Folate Receptor 1, Adult (FOLR1) PAB233Hu01 Cyclin Dependent Kinase 4 (CDK4) PAA150Hu04 Carcinoembryonic Antigen (CEA) PAB905Hu01 Interleukin 7 Receptor (IL7R) PAC823Hu01 Thymidine Kinase 1, Soluble (TK1) PAH838Hu01 Isocitrate Dehydrogenase 2, mitochondrial (IDH2) PAK078Mu01 Fas Associating Death Domain Containing Protein (FADD) PAA537Hu01 Enolase, Neuron Specific (NSE) PAA651Hu01 Hyaluronan Binding Protein 1 (HABP1) PAB215Hu02 Fibrinogen Beta (FGb) PAB769Hu01 S100 Calcium Binding Protein A6 (S100A6) PAB231Hu01 Keratin 18 (KRT18) PAH839Hu01 Isocitrate Dehydrogenase 1, Soluble (IDH1) PAE748Hu01 Karyopherin Alpha 2 (KPNa2) PAB081Hu02 Heat Shock 70kDa Protein 1A (HSPA1A) PAA778Mu01 B-Cell Leukemia/Lymphoma 2 (Bcl2) PAA853Hu03 Caspase 8 (CASP8) PAA399Mu01 High Mobility Group Protein 1 (HMG1) PAA303Mu01 Galectin 3 (GAL3) PAA537Mu02 Enolase, Neuron Specific (NSE) PAA994Ra01 Acid Phosphatase 1 (ACP1) PAB083Ra01 Superoxide Dismutase 2, Mitochondrial (SOD2) PAB449Mu01 Enolase, Non Neuronal (NNE) PAA376Mu01 Actinin Alpha 2 (ACTN2) PAA553Ra01 Matrix Metalloproteinase 9 (MMP9) PAA929Bo01 Retinol Binding Protein 4, Plasma (RBP4) PAA491Ra02 Keratin 2 (KRT2) PAC025Hu01 Keratin 8 (KRT8) PAB231Mu01 Keratin 18 (KRT18) PAC598Hu03 Vanin 1 (VNN1) -

Phase I Study of Recombinant Human Interleukin-7 Administration in Subjects with Refractory Malignancy
Published OnlineFirst January 12, 2010; DOI: 10.1158/1078-0432.CCR-09-1303 Cancer Therapy: Clinical Clinical Cancer Research Phase I Study of Recombinant Human Interleukin-7 Administration in Subjects with Refractory Malignancy Claude Sportès1, Rebecca R. Babb1, Michael C. Krumlauf1, Frances T. Hakim1, Seth M. Steinberg2, Catherine K. Chow5, Margaret R. Brown6, Thomas A. Fleisher6, Pierre Noel6, Irina Maric6, Maryalice Stetler-Stevenson3, Julie Engel7, Renaud Buffet7, Michel Morre7, Robert J. Amato8, Andrew Pecora9, Crystal L. Mackall4, and Ronald E. Gress1 Abstract Purpose: Interleukin-7 (IL-7) has critical and nonredundant roles in T-cell development, hematopoi- esis, and postdevelopmental immune functions as a prototypic homeostatic cytokine. Based on a large body of preclinical evidence, it may have multiple therapeutic applications in immunodeficiency states, either physiologic (immunosenescence), pathologic (HIV), or iatrogenic (postchemotherapy and posthe- matopoietic stem cell transplant), and may have roles in immune reconstitution or enhancement of im- munotherapy. We report here on the toxicity and biological activity of recombinant human IL-7 (rhIL-7) in humans. Design: Subjects with incurable malignancy received rhIL-7 subcutaneously every other day for 2 weeks in a phase I interpatient dose escalation study (3, 10, 30, and 60 μg/kg/dose). The objectives were safety and dose-limiting toxicity determination, identification of a range of biologically active doses, and char- acterization of biological and, possibly, antitumor effects. Results: Mild to moderate constitutional symptoms, reversible spleen and lymph node enlargement, and marked increase in peripheral CD3+, CD4+, and CD8+ lymphocytes were seen in a dose-dependent and age-independent manner in all subjects receiving ≥10 μg/kg/dose, resulting in a rejuvenated circulat- ing T-cell profile, resembling that seen earlier in life. -

Evolutionary Divergence and Functions of the Human Interleukin (IL) Gene Family Chad Brocker,1 David Thompson,2 Akiko Matsumoto,1 Daniel W
UPDATE ON GENE COMPLETIONS AND ANNOTATIONS Evolutionary divergence and functions of the human interleukin (IL) gene family Chad Brocker,1 David Thompson,2 Akiko Matsumoto,1 Daniel W. Nebert3* and Vasilis Vasiliou1 1Molecular Toxicology and Environmental Health Sciences Program, Department of Pharmaceutical Sciences, University of Colorado Denver, Aurora, CO 80045, USA 2Department of Clinical Pharmacy, University of Colorado Denver, Aurora, CO 80045, USA 3Department of Environmental Health and Center for Environmental Genetics (CEG), University of Cincinnati Medical Center, Cincinnati, OH 45267–0056, USA *Correspondence to: Tel: þ1 513 821 4664; Fax: þ1 513 558 0925; E-mail: [email protected]; [email protected] Date received (in revised form): 22nd September 2010 Abstract Cytokines play a very important role in nearly all aspects of inflammation and immunity. The term ‘interleukin’ (IL) has been used to describe a group of cytokines with complex immunomodulatory functions — including cell proliferation, maturation, migration and adhesion. These cytokines also play an important role in immune cell differentiation and activation. Determining the exact function of a particular cytokine is complicated by the influence of the producing cell type, the responding cell type and the phase of the immune response. ILs can also have pro- and anti-inflammatory effects, further complicating their characterisation. These molecules are under constant pressure to evolve due to continual competition between the host’s immune system and infecting organisms; as such, ILs have undergone significant evolution. This has resulted in little amino acid conservation between orthologous proteins, which further complicates the gene family organisation. Within the literature there are a number of overlapping nomenclature and classification systems derived from biological function, receptor-binding properties and originating cell type. -

Illuminating the Source of in Vivo Interleukin-7
DOI 10.4110/in.2011.11.1.1 REVIEW ARTICLE pISSN 1598-2629 eISSN 2092-6685 Seeing Is Believing: Illuminating the Source of In Vivo Interleukin-7 Grace Yoonhee Kim1,2,3, Changwan Hong1 and Jung-Hyun Park1* 1Experimental Immunology Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD, 2Howard Hughes Medical Institute-NIH Research Scholars Program, Bethesda, MD, 3The Johns Hopkins University School of Medicine, Baltimore, MD, USA Interleukin-7 (IL-7) is an essential cytokine for T cells. INTRODUCTION However, IL-7 is not produced by T cells themselves such that T cells are dependent on extrinsic IL-7. In fact, in the ab- Originally described as a pre-B cell growth factor expressed sence of IL-7, T cell development in the thymus as well as in bone marrow stromal cells (1), interleukin-7 (IL-7) was survival of naive T cells in the periphery is severely impaired. subsequently identified as an essential and non-redundant cy- Furthermore, modulating IL-7 availability in vivo either by ge- tokine in T cells (2,3). In fact, IL-7 plays a critical role in netic means or other experimental approaches determines every aspect of T cell biology, including early T cell develop- the size, composition and function of the T cell pool. ment in the thymus, CD4/CD8 lineage choice during positive Consequently, understanding IL-7 expression is critical for understanding T cell immunity. Until most recently, however, selection as well as maintaining the survival and homeostasis the spatiotemporal expression of in vivo IL-7 has remained of naïve and memory T cells in the periphery (2-6). -

Increased Expression of TLR4 in Circulating CD4+T Cells in Patients with Allergic Conjunctivitis and in Vitro Attenuation of Th2 Inflammatory Response by Alpha-MSH
International Journal of Molecular Sciences Article Increased Expression of TLR4 in Circulating CD4+T Cells in Patients with Allergic Conjunctivitis and In Vitro Attenuation of Th2 Inflammatory Response by Alpha-MSH Jane E. Nieto 1, Israel Casanova 1, Juan Carlos Serna-Ojeda 2, Enrique O. Graue-Hernández 2, Guillermo Quintana 1, Alberto Salazar 1 and María C. Jiménez-Martinez 1,3,* 1 Department of Immunology and Research Unit, Institute of Ophthalmology “Conde de Valenciana Foundation”, Ciudad de México 06800, Mexico; [email protected] (J.E.N.); [email protected] (I.C.); [email protected] (G.Q.); [email protected] (A.S.) 2 Department of Cornea and Refractive Surgery, Institute of Ophthalmology “Conde de Valenciana Foundation”, Ciudad de México 06800, Mexico; [email protected] (J.C.S.-O.); [email protected] (E.O.G.-H.) 3 Department of Biochemistry, Faculty of Medicine, National Autonomous University of Mexico, Ciudad de México 04510, Mexico * Correspondence: [email protected]; Tel.: +52-(55)-5442170 Received: 16 September 2020; Accepted: 21 October 2020; Published: 23 October 2020 Abstract: Ocular allergic diseases are frequently seen in ophthalmological clinical practice. Immunological damage is mediated by a local Th2 inflammatory microenvironment, accompanied by changes in circulating cell subsets, with more effector cells and fewer T regulatory cells (Tregs). This study aimed to evaluate the involvement of toll-like receptor 4 (TLR4) and α-melanocyte stimulating hormone (α-MSH) in the immune regulation associated with perennial allergic conjunctivitis (PAC). We performed an Ag-specific stimulation during 72 h of culturing with or without lipopolysaccharide (LPS) or α-MSH in peripheral blood mononuclear cells (PBMC), analyzing the cell subsets and cytokines induced by the stimuli.