Mechanism of Nitration of Phenolsulphon1c Acids
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemicals in the Fourth Report and Updated Tables Pdf Icon[PDF
Chemicals in the Fourth National Report on Human Exposure to Environmental Chemicals: Updated Tables, March 2021 CDC’s Fourth National Report on Human Exposure to Environmental Chemicals: Updated Tables, March 2021 provides exposure data on the following chemicals or classes of chemicals. The Updated Tables contain cumulative data from national samples collected beginning in 1999–2000 and as recently as 2015-2016. Not all chemicals were measured in each national sample. The data tables are available at https://www.cdc.gov/exposurereport. An asterisk (*) indicates the chemical has been added since publication of the Fourth National Report on Human Exposure to Environmental Chemicals in 2009. Adducts of Hemoglobin Acrylamide Formaldehyde* Glycidamide Tobacco Alkaloids and Metabolites Anabasine* Anatabine* Cotinine Cotinine-n-oxide* Hydroxycotinine* Trans-3’-hydroxycotinine* 1-(3-Pyridyl)-1-butanol-4-carboxylic acid* Nicotine* Nicotine-N’-oxide* Nornicotine* Tobacco-Specific Nitrosamines (TSNAs) N’-Nitrosoanabasine (NAB)* N’-Nitrosoanatabine (NAT)* N’-Nitrosonornicotine (NNN)* Total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol) (NNAL)* Volatile N-nitrosamines (VNAs) N-Nitrosodiethylamine (NDEA)* N-Nitrosoethylmethylamine (NMEA)* N-Nitrosomorpholine (NMOR)* N-Nitrosopiperidine (NPIP)* N-Nitrosopyrrolidine (NPYR)* Disinfection By-Products Bromodichloromethane Dibromochloromethane Tribromomethane (Bromoform) Trichloromethane (Chloroform) Personal Care and Consumer Product Chemicals and Metabolites Benzophenone-3 Bisphenol A Bisphenol F* Bisphenol -

Method 3640A
METHOD 3640A GEL-PERMEATION CLEANUP 1.0 SCOPE AND APPLICATION 1.1 Gel-permeation chromatography (GPC) is a size exclusion cleanup procedure using organic solvents and hydrophobic gels in the separation of synthetic macromolecules (1). The packing gel is porous and is characterized by the range or uniformity (exclusion range) of that pore size. In the choice of gels, the exclusion range must be larger than the molecular size of the molecules to be separated (2). A cross-linked divinylbenzene-styrene copolymer (SX-3 Bio Beads or equivalent) is specified for this method. 1.2 General cleanup application - GPC is recommended for the elimination from the sample of lipids, polymers, copolymers, proteins, natural resins and polymers, cellular components, viruses, steroids, and dispersed high-molecular- weight compounds (2). GPC is appropriate for both polar and non-polar analytes, therefore, it can be effectively used to cleanup extracts containing a broad range of analytes. 1.3 Specific application - This method includes guidance for cleanup of sample extracts containing the following analytes from the RCRA Appendix VIII and Appendix IX lists: ______________________________________________________________________________ Compound Name CAS No.a ______________________________________________________________________________ Acenaphthene 83-32-9 Acenaphthylene 208-96-8 Acetophenone 98-86-2 2-Acetylaminofluorene 53-96-3 Aldrin 309-00-2 4-Aminobiphenyl 92-67-1 Aniline 62-53-3 Anthracene 120-12-7 Benomyl 17804-35-2 Benzenethiol 108-98-5 Benzidine 92-87-5 -

Amidation of Phenol Derivatives: a Direct Synthesis of Paracetamol (Acetaminophen) from Hydroquinone
Amidation of phenol derivatives : a direct synthesis of paracetamol (acetaminophen) from hydroquinone. R. Joncour, N. Duguet, E. Métay, A. Ferreira, M. Lemaire To cite this version: R. Joncour, N. Duguet, E. Métay, A. Ferreira, M. Lemaire. Amidation of phenol derivatives : a direct synthesis of paracetamol (acetaminophen) from hydroquinone.. Green Chemistry, Royal Society of Chemistry, 2014, 16, pp.2997-3002. 10.1039/C4GC00166D. hal-01149158 HAL Id: hal-01149158 https://hal.archives-ouvertes.fr/hal-01149158 Submitted on 7 Jun 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Amidation of phenol derivatives: a direct synthesis of paracetamol (acetaminophen) from hydroquinone a a a b a Roxan Joncour, Nicolas Duguet, Estelle Métay, Amadéo Ferreira and Marc Lemaire* A direct synthesis of paracetamol (acetaminophen) from technologies have not found their place yet. Over the last century, hydroquinone has been developed using ammonium acetate many routes have been explored for the paracetamol production but as amidating agent. The reaction proceeds in acetic acid at all those which have emerged industrially are based on the elevated temperature without any metallic catalyst. Under acetylation of para-aminophenol (PAP) as final stage (Scheme 2). -

2019 Minnesota Chemicals of High Concern List
Minnesota Department of Health, Chemicals of High Concern List, 2019 Persistent, Bioaccumulative, Toxic (PBT) or very Persistent, very High Production CAS Bioaccumulative Use Example(s) and/or Volume (HPV) Number Chemical Name Health Endpoint(s) (vPvB) Source(s) Chemical Class Chemical1 Maine (CA Prop 65; IARC; IRIS; NTP Wood and textiles finishes, Cancer, Respiratory 11th ROC); WA Appen1; WA CHCC; disinfection, tissue 50-00-0 Formaldehyde x system, Eye irritant Minnesota HRV; Minnesota RAA preservative Gastrointestinal Minnesota HRL Contaminant 50-00-0 Formaldehyde (in water) system EU Category 1 Endocrine disruptor pesticide 50-29-3 DDT, technical, p,p'DDT Endocrine system Maine (CA Prop 65; IARC; IRIS; NTP PAH (chem-class) 11th ROC; OSPAR Chemicals of Concern; EuC Endocrine Disruptor Cancer, Endocrine Priority List; EPA Final PBT Rule for 50-32-8 Benzo(a)pyrene x x system TRI; EPA Priority PBT); Oregon P3 List; WA Appen1; Minnesota HRV WA Appen1; Minnesota HRL Dyes and diaminophenol mfg, wood preservation, 51-28-5 2,4-Dinitrophenol Eyes pesticide, pharmaceutical Maine (CA Prop 65; IARC; NTP 11th Preparation of amino resins, 51-79-6 Urethane (Ethyl carbamate) Cancer, Development ROC); WA Appen1 solubilizer, chemical intermediate Maine (CA Prop 65; IARC; IRIS; NTP Research; PAH (chem-class) 11th ROC; EPA Final PBT Rule for 53-70-3 Dibenzo(a,h)anthracene Cancer x TRI; WA PBT List; OSPAR Chemicals of Concern); WA Appen1; Oregon P3 List Maine (CA Prop 65; NTP 11th ROC); Research 53-96-3 2-Acetylaminofluorene Cancer WA Appen1 Maine (CA Prop 65; IARC; IRIS; NTP Lubricant, antioxidant, 55-18-5 N-Nitrosodiethylamine Cancer 11th ROC); WA Appen1 plastics stabilizer Maine (CA Prop 65; IRIS; NTP 11th Pesticide (EPA reg. -

Environmental Protection Agency 40 CFR Parts 63, 268, Et Al
Tuesday, June 14, 2005 Part II Environmental Protection Agency 40 CFR Parts 63, 268, et al. Waste Management System; Testing and Monitoring Activities; Final Rule: Methods Innovation Rule and SW–846 Final Update IIIB; Final Rule VerDate jul<14>2003 20:19 Jun 13, 2005 Jkt 205001 PO 00000 Frm 00001 Fmt 4717 Sfmt 4717 E:\FR\FM\14JNR2.SGM 14JNR2 34538 Federal Register / Vol. 70, No. 113 / Tuesday, June 14, 2005 / Rules and Regulations ENVIRONMENTAL PROTECTION should make it easier and more cost I. General Information AGENCY effective to comply with the affected regulations, without compromising A. Does This Action Apply to Me? 40 CFR Parts 63, 258, 260, 261, 264, human health or environmental You may be covered by this action if 265, 266, 268, 270, 271, and 279 protection. you conduct waste sampling and [RCRA–2002–0025; FRL–7916–1] DATES: This final rule is effective on July analysis for Resource Conservation and RIN 2050–AE41 14, 2005. The incorporation by reference Recovery Act (RCRA)-or National of certain publications listed in the rule Emission Standards for Hazardous Air Waste Management System; Testing is approved by the Director of the Pollutants (NESHAP)-related activities. and Monitoring Activities; Final Rule: Federal Register as of July 14, 2005. Covered entities include anyone who Methods Innovation Rule and SW–846 ADDRESSES: EPA has established a generates, treats, stores, or disposes of Final Update IIIB docket for this action under Docket ID hazardous or nonhazardous solid waste No. RCRA–2002–0025. All documents and is subject to RCRA subtitle C or D AGENCY: Environmental Protection in the docket are listed in the EDOCKET sampling and analysis requirements; Agency (EPA). -

Dissociation Constants of Some Substituted Nitrophenols in Aqueous Solution at 25 Degrees C
JOU RNAL OF RESEARCH of the National Bureau of Standards - A. Physics and Chemistry Vol. 71 A, No.5, September-October 1967 . Dissociation Constants of Some Substituted Nitrophenols In Aqueous Solution at 2S 0 C R. A. Robinson Institute for Materials Research, National Bureau of Standards, Washington, D.C. 20234 (May 9, 1967) The dissociation constants of twelve substituted phenols with a nitro group in the 0- or p-position have been determined by spectrophotometric measurements in aqueous solution at 25°C. Key Words: Dissociation constant, nitrophenols, substituted phenols. 1. Introduction 2,4-Dinitro-6-methylphenol was recrystalli2;p.d twice from benzene-cyclohexane mp 86.0-86.7 °C; 2,6-dini The determination of the dissociation constants of tro-4-methylphenol from benzene-cyclohexane and tnen a number of substituted phenols by the spectrophoto from cyclohexane, mp 80.7- 81.2 °C; 2,4-dinitro-3- metric method has been described recently [1, 2, 3, 4, methyl-6-isopropylphenol from aqueous ethanol and 5, 6, 7).1 The purpose of this paper is to present data th en from li groin , mp 55.0-55_7 °C. 2,4-Dinitro-6- for twelve substituted phenols with an indicator phenyl phenol recrystallized from methyl ethyl ketone range pH 2.1-5.5. and th en from ethyl acetate, mp 206.6-207.0 °C; 2,4-dinitro-6-isopropylphenol from 95 percent ethanol and then from hexane, mp 54.0-55.0 0c. 2. Experimental Procedure Table 1 gives some characteristics of the absorption spectra of these compounds, recording the wavelengths 2.1 . -

RED) Paranitrophenol UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON, D.C
United States Prevention, Pesticides EPA 738-R-97-016 Environmental Protection And Toxic Substances January 1998 Agency (7508W) Reregistration Eligibility Decision (RED) Paranitrophenol UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON, D.C. 20460 OFFICE OF PREVENTION, PESTICIDES AND TOXIC SUBSTANCES CERTIFIED MAIL Dear Registrant: I am pleased to announce that the Environmental Protection Agency has completed its reregistration eligibility review and decisions on the pesticide Paranitrophenol. The enclosed Reregistration Eligibility Decision (RED) contains the Agency's evaluation of the data base of this chemical, its conclusions on the potential human health and environmental risks of the current product uses, and decisions and conditions under which these uses and product can be used. The RED includes the data and labeling requirements for the product reregistration decision. To assist you with a proper response, read the enclosed document entitled "Summary of Instructions for Responding to the RED.” The required response is due 90 days from the receipt of this letter. Complete and timely responses will avoid the Agency taking the enforcement action of suspension against your product. If you have questions on the labeling language for Paranitrophenol-containing product requirements or wish to meet with the Agency, please contact the Special Review and Reregistration Division representative Veronica Dutch (703) 308-8585. Sincerely yours, Lois Rossi, Director Special Review and Reregistration Division Enclosures SUMMARY OF INSTRUCTIONS FOR RESPONDING TO THE REREGISTRATION ELIGIBILITY DECISION (RED) 1. APPLICATION FOR REREGISTRATION Decision--You must submit the following items for each product within ninety days of the date of this letter (RED issuance date). a. Application for Reregistration (EPA Form 8570-1). -

Chem 263 Oct 13, 2016
Chem 263 Oct 13, 2016 Acidity of alcohols continued … Cl NO2 NO2 OH OH OH OH OH cyclohexanol phenol 4-chlorophenol 4-nitrophenol 3-nitrophenol pKa = 18 pKa = 10 pKa = 9.3 pKa = 7 pKa = 9.3 NO2 NO2 O2N O2N NO2 OH OH 2,4-dinitrophenol 2,4,6-trinitrophenol pKa = 4.5 picric acid pKa = 0.5 What is the influence of substituents? inductive & resonance 8 As the pKa values above show, phenol is 10 more acidic than cyclohexanol. Phenol has a pKa value of 10 (given this information, you should immediately recognize that it is more much acidic (about 6 orders of magnitude) than water (pKa 15.7) and methanol (pKa 16) since it has lower pKa value). As phenol is more acidic, this means that its conjugate anion is more stable. The phenoxide anion is stabilized through resonance (shown above). It has 4 resonance forms, and therefore, more ability to spread the negative charge and be stabilized. Where does the equilibrium lie for ionization of phenol to phenoxide and a proton (H+) ? Answer: It lies far to the left (not ionized). Even though phenol is 106 more acidic than -10 10 water, its pKa of 10 tells you that the acidity constant is 10 or that only one part in 10 is ionized; the rest exists as phenol with H attached to oxygen. Example: Chlorophenol Cl Cl Cl O Cl O O O Is the anion more or less stabilized compared to phenol? Answer: More stabilized. The chlorine atom is electron withdrawing, and stabilizes the negative charge at the para position through the inductive withdrawing effect. -

Chemical Compatibility Storage Group
CHEMICAL SEGREGATION Chemicals are to be segregated into 11 different categories depending on the compatibility of that chemical with other chemicals The Storage Groups are as follows: Group A – Compatible Organic Acids Group B – Compatible Pyrophoric & Water Reactive Materials Group C – Compatible Inorganic Bases Group D – Compatible Organic Acids Group E – Compatible Oxidizers including Peroxides Group F– Compatible Inorganic Acids not including Oxidizers or Combustible Group G – Not Intrinsically Reactive or Flammable or Combustible Group J* – Poison Compressed Gases Group K* – Compatible Explosive or other highly Unstable Material Group L – Non-Reactive Flammable and Combustible, including solvents Group X* – Incompatible with ALL other storage groups The following is a list of chemicals and their compatibility storage codes. This is not a complete list of chemicals, but is provided to give examples of each storage group: Storage Group A 94‐75‐7 2,4‐D (2,4‐Dichlorophenoxyacetic acid) 94‐82‐6 2,4‐DB 609-99-4 3,5-Dinitrosalicylic acid 64‐19‐7 Acetic acid (Flammable liquid @ 102°F avoid alcohols, Amines, ox agents see SDS) 631-61-8 Acetic acid, Ammonium salt (Ammonium acetate) 108-24-7 Acetic anhydride (Flammable liquid @102°F avoid alcohols see SDS) 79‐10‐7 Acrylic acid Peroxide Former 65‐85‐0 Benzoic acid 98‐07‐7 Benzotrichloride 98‐88‐4 Benzoyl chloride 107-92-6 Butyric Acid 115‐28‐6 Chlorendic acid 79‐11‐8 Chloroacetic acid 627‐11‐2 Chloroethyl chloroformate 77‐92‐9 Citric acid 5949-29-1 Citric acid monohydrate 57-00-1 Creatine 20624-25-3 -
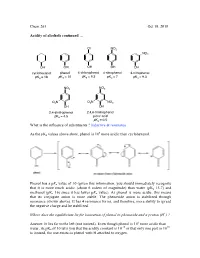
Chem 263 Oct 19 Revised
Chem 263 Oct 19, 2010 Acidity of alcohols continued … Cl NO2 NO2 OH OH OH OH OH cyclohexanol phenol 4-chlorophenol 4-nitrophenol 3-nitrophenol pKa = 18 pKa = 10 pKa = 9.3 pKa = 7 pKa = 9.3 NO2 NO2 O2N O2N NO2 OH OH 2,4-dinitrophenol 2,4,6-trinitrophenol pKa = 4.5 picric acid pKa = 0.5 What is the influence of substituents ? inductive & resonance 8 As the pKa values above show, phenol is 10 more acidic than cyclohexanol. Phenol has a pKa value of 10 (given this information, you should immediately recognize that it is more much acidic (about 6 orders of magnitude) than water (pKa 15.7) and methanol (pKa 16) since it has lower pKa value). As phenol is more acidic, this means that its conjugate anion is more stable. The phenoxide anion is stabilized through resonance (shown above). It has 4 resonance forms, and therefore, more ability to spread the negative charge and be stabilized. Where does the equilibrium lie for ionization of phenol to phenoxide and a proton (H+) ? Answer: It lies far to the left (not ionized). Even though phenol is 106 more acidic than -10 10 water, its pKa of 10 tells you that the acidity constant is 10 or that only one part in 10 is ionized, the rest exists as phenol with H attached to oxygen. Example: Chlorophenol (Above) Cl Cl Cl O Cl O O O Is the anion more or less stabilized compared to phenol? Answer: more stabilized. The chlorine atom is electron withdrawing, and stabilizes the negative charge at the para position through the inductive withdrawing effect. -

57518 Federal Register / Vol. 61, No. 216 / Wednesday, November 6, 1996 / Rules and Regulations
57518 Federal Register / Vol. 61, No. 216 / Wednesday, November 6, 1996 / Rules and Regulations ENVIRONMENTAL PROTECTION implement certain pollution prevention, (BPT), §§ 455.43 and 455.63 (BCT), and AGENCY recycle and reuse practices. Facilities §§ 455.44 and 455.64 (BAT) are choosing and implementing the established in the National Pollutant 40 CFR Part 455 pollution prevention alternative will Discharge Elimination System (NPDES) receive a discharge allowance. permits. [FRL±5630±9] The final rule will benefit the ADDRESSES: For additional technical RIN 2040±AC21 environment by removing toxic information write to Ms. Shari H. pollutants (pesticide active ingredients Zuskin, Engineering & Analysis Division Pesticide Chemicals Category, and priority pollutants) from water (4303), U.S. EPA, 401 M Street SW, Formulating, Packaging and discharges that have adverse effects on Washington, D.C. 20460 or send e-mail Repackaging Effluent Limitations human health and aquatic life. EPA has to: [email protected] or call Guidelines, Pretreatment Standards, estimated the compliance costs and at (202) 260±7130. For additional and New Source Performance economic impacts expected to result economic information contact Dr. Lynne Standards from the Zero Discharge/Pollution Tudor at the address above or by calling Prevention Alternative (i.e., Zero/P2 AGENCY: Environmental Protection (202) 260±5834. Agency. Alternative). The Agency has determined that the Zero/P2 Alternative The complete record (excluding ACTION: Final rule. will result in a similar removal of toxic confidential business information) for this rulemaking is available for review SUMMARY: This final regulation limits pound equivalents per year at EPA's Water Docket; 401 M Street, the discharge of pollutants into (approximately 7.6 million toxic pound SW, Washington, DC 20460. -

Toxicological Profile for Nitrophenols: 2-Nitrophenol 4-Nitrophenol
TOXICOLOGICAL PROFILE FOR NITROPHENOLS: 2-NITROPHENOL 4-NITROPHENOL Agency for Toxic Substances and Disease Registry U.S. Public Health Service July 1992 ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry. 1 1. PUBLIC HEALTH STATEMENT This Statement was prepared to give you information about 2-nitrophenol and 4-nitrophenol and to emphasize the human health effects that may result from exposure to them. The Environmental Protection Agency (EPA) has identified 1,177 National Priorities List (NPL) sites. Nitrophenols have been found at 14 of these sites. However, we do not know how many of the 1,177 NPL sites have been evaluated for 2-nitrophenol and 4-nitrophenol. As EPA evaluates more sites, the number of sites at which nitrophenols are found may change. This information is important for you because nitrophenols may cause harmful effects and because these sites are potential or actual sources of human exposure to 2-nitrophenol and 4-nitrophenol. When a chemical is released from a large area, such as an industrial plant, or from a container, such as a drum or bottle, it enters the environment as a chemical emission. This emission, which is also called a release, does not always lead to exposure. You can be exposed to a chemical only when you come into contact with it. You may be exposed to it in the environment by breathing, eating, or drinking substances containing the chemical, or from skin contact with it. If you are exposed to a hazardous substance such as nitrophenols, several factors will determine whether harmful health effects will occur and what the type and severity of those health effects will be.