COVID-19 Response Plan for Assisted Living Facilities
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Minne-ALF Project Overview and Retro-Fit Dowel Study Results
Final Report 2000-02 Minne-ALF Project Overview and Retro-Fit Dowel Study Results Technical Report Documentation Page 1. Report No. 2. 3. Recipient’s Accession No. MN/RC - 2000-02 4. Title and Subtitle 5. Report Date MINNE-ALF PROJECT OVERVIEW AND RETRO-FIT December 1999 DEWEL STUDY RESULTS 6. 7. Author(s) 8. Performing Organization Report No. Rebecca A. Embacher Mark B. Snuyder 9. Performing Organization Name and Address 10. Project/Task/Work Unit No. University of Minnesota Civil Engineering Department 11. Contract (C) or Grant (G) No. 500 Pillsbury Drive, S.E. Minneapolis, MN 55455 c)72272 wo) 146 12. Sponsoring Organization Name and Address 13. Type of Report and Period Covered Minnesota Department of Transportation Final Report 1994-1999 395 John Ireland Boulevard Mail Stop 330 St. Paul, Minnesota 55155 14. Sponsoring Agency Code 15. Supplementary Notes 16. Abstract (Limit: 200 words) A laboratory-based linear loading pavement test stand, the Minnesota Accelerated Loading Facility (Minne-ALF) simulates the passage of heavy traffic loads moving at speeds up to 65 kph (40 mph) over small, full-scale pavement test slabs. Hydraulic actuators control a rocker beam, which simulates loads. Researchers simulated the passage of 40-KN (9-kip) single-wheel loads at a rate of 172,000 per day, although wheel loads up to 100 kN (22 kips) can be simulated at varying speeds. Full-axle simulations are possible with frame modifications. Concrete slabs were cast and dowels were installed in slots across crack/joints. Test variables included joint fact texture, repair backfill material, and dowel material and length. -

Making Care Count: an Overview of the Women's Economic
Making care count AN OVERVIEW OF THE WOMEN’S ECONOMIC EMPOWERMENT AND CARE INITIATIVE ABOUT THE COVER. Created by African artist Monica Obaga, this artwork represents a vision of a future where families share care work at home. It was first used for an online campaign during the celebration of the African Women’s Day in July 2020. Across the globe, unpaid care and domestic work (UCDW) sustains communities and economies, provides essential care for children, sick and elderly people and those living with disabilities, and keeps households clean and families fed. Without unpaid care, the global economy as we know it would grind to a halt. Yet this work falls disproportionately on women and girls, limiting their opportunities to participate in decent paid employment, education, leisure and political life. Heavy and unequal UCDW traps women and girls in cycles of poverty and stops them from being part of solutions. Making unpaid care more valued and more visible In low-income settings such as Zimbabwe and From ‘radical’ to mainstream: the Philippines, where essential public services the evolving discussion on unpaid care are inadequate and tasks such as collecting Conversations on UCDW have evolved over the water and firewood are particularly heavy and decades from the ‘domestic labour debate’ time-consuming, women do up to six times of the 1970s—then considered radical—to as much UCDW as men.1 When domestic tasks addressing care increasingly being seen require inordinately long hours, time for direct today as a precondition for women’s political, care of people is further constrained.2 economic and social empowerment. -

Erformance Testing for Superpave and Structural Validation
Performance Testing for Superpave and Structural Validation PUBLICATION NO. FHWA-HRT-11-045 NOVEMBER 2012 Research, Development, and Technology Turner-Fairbank Highway Research Center 6300 Georgetown Pike McLean, VA 22101-2296 FOREWORD This final report provides the comprehensive findings from two Transportation Pooled Fund (TPF) research projects, TPF-5(019): Full-Scale Accelerated Performance Testing for Superpave and Structural Validation and SPR-2(174): Accelerated Pavement Testing of Crumb Rubber Modified Asphalt Pavements. The research identified candidate purchase specification tests for asphalt binder that better discriminate expected fatigue cracking and rutting performance than current SUperior PERforming Asphalt PAVEment (Superpave®) tests. Full-scale accelerated pavement testing and laboratory characterization tests on mixtures and binders provided the basis for the recommendations. This report documents a historical review of the development of asphalt binder performance specifications, experimental design, test pavement construction and performance, statistical methodology to rank and identify the strongest candidates, and all pertinent laboratory characterization of binders and mixtures that supplemented the recommendations. The research also provided a detailed case study of pavement evaluation using falling weight deflectometer and objective means to evaluate two emerging technologies; the asphalt mixture performance tester and the Mechanistic-Empirical Pavement Design Guide.(1) This document will be of interest to highway personnel involved with Superpave®, materials selection, performance specifications, and pavement design and evaluation. Jorge E. Pagán-Ortiz Director, Office of Infrastructure Research and Development Notice This document is disseminated under the sponsorship of the U.S. Department of Transportation in the interest of information exchange. The U.S. Government assumes no liability for the use of the information contained in this document. -

AMF / ALF SERIES Albedometer Mounting and Levelling Fixtures: Combine 2 Pyranometers Into 1 Albedometer with Our AMF03 and AMF02 Albedometer Kits
Hukseflux Thermal Sensors ACCESSORY AMF / ALF SERIES Albedometer mounting and levelling fixtures: combine 2 pyranometers into 1 albedometer with our AMF03 and AMF02 albedometer kits Hukseflux offers a practical range of mounting and levelling fixtures to construct albedometers from its popular pyranometers and make installation and levelling easy. Albedometers are increasingly popular in bifacial PV module monitoring. AMF03 allows you to combine two SR30-M2-D1 spectrally flat Class A pyranometers or two SR15 series Class B pyranometers into one albedometer. AMF02 does so for two SR11 or two SR20 series pyranometers. The modular design facilitates maintenance and calibration of the pyranometers. Both albedometer kits include a mounting fixture and a glare screen. ALF01 is a levelling fixture that may be combined with AMF03, AMF02 or SRA series albedometers, and helps levelling the instrument. Hukseflux offers a full range of dedicated, ready- Introduction to-use albedometers for measuring albedo. Albedo, also called solar reflectance, is defined as Alternatively, with the AMF03 or AMF02 mounting the ratio of the reflected to the global radiation. fixture in AMF / ALF series, you may choose to The solar albedo depends on the directional construct albedometers from popular Hukseflux distribution of incoming radiation and on surface pyranometers yourself. AMF03, AMF02 and SRA properties at ground level. Albedos of typical series albedometers can be levelled with ALF01 surfaces range from about 4 % for fresh asphalt, levelling fixture. and 15 % for green grass to 90 % for fresh snow. An albedometer is an instrument composed of two pyranometers, the upfacing one measuring global solar radiation, the downfacing one measuring reflected solar radiation. -

Muere Max Wright, El «Padre» Humano De ALF
Muere Max Wright, el «padre» humano de ALF Max Wright, uno de los protagonistas de la popular serie de televisión de los años 80 ALF, falleció este miércoles. Familiares confirmaron que el actor, quien interpretó a Willy Tanner, el padre de la familia que acogió al peludo extraterrestre, murió en su casa en Hermosa Beach, a las afueras de Los Ángeles, California. Wright, que tenía 75 años, libró una batalla contra el cáncer durante muchos años. Al actor, nacido en Detroit (Michigan), le fue diagnosticado un linfoma en 1995, que permaneció en estado de remisión durante mucho tiempo. La esposa del artista, Linda Ybarrondo, con quien se había casado en 1965 y tenía dos hijos, falleció en 2017 a raíz de un cáncer de mama. Wright ya había sido noticia hace años por sus excesos. Drogándose sin parar, fue pillado por fotógrafos en un fumadero de crack e incluso se filtraron imágenes en las que mantenía sexo homosexual en una orgía mientras se consumían varias drogas. Además de actuar en cuatro temporadas de Alf, Wright participó en programas como Buffalo Bill, Cheers, Misfits of Science, Dudley y The Norm Show, recordó la publicación. El intérprete también hizo parte de películas como All That Jazz, Reds, The Sting II, Soul Man, The Shadow, entre otras. Con cuatro temporadas y 103 episodios emitidos entre 1986 y 1990, ALF giraba en torno a la llegada de un simpático extraterrestre a la vida de una familia ordinaria y fue un hito de la televisión estadounidense de los años 80. En agosto del año pasado, el portal especializado TVLine informó que la productora Warner Bros Television planteaba recuperar ALF. -

Rules for Level I and II Assisted Living
100 SCOPE ...........................................................................................................................................................3 200 PURPOSE......................................................................................................................................................3 300 DEFINITIONS ..............................................................................................................................................3 400 LICENSURE ...............................................................................................................................................10 401 LICENSING INFORMATION.....................................................................................................................11 402 INITIAL LICENSURE .................................................................................................................................12 403 COMPLIANCE.............................................................................................................................................12 404 APPLICATION, EXPIRATION AND RENEWAL OF LICENSE .............................................................12 405 CHANGE IN OWNERSHIP.........................................................................................................................15 500 ADMINISTRATION ..................................................................................................................................16 501 GOVERNING BODY...................................................................................................................................16 -

The Finding Aid to the Alf Evers Archive
FINDING AID TO THE ALF EVERS’ ARCHIVE A Account books & Ledgers Ledger, dark brown with leather-bound spine, 13 ¼ x 8 ½”: in front, 15 pp. of minutes in pen & ink of meetings of officers of Oriental Manufacturing Co., Ltd., dating from 8/9/1898 to 9/15/1899, from its incorporation to the company’s sale; in back, 42 pp. in pencil, lists of proverbs; also 2 pages of proverbs in pencil following the minutes Notebook, 7 ½ x 6”, sold by C.W. & R.A. Chipp, Kingston, N.Y.: 20 pp. of charges & payments for goods, 1841-52 (fragile) 20 unbound pages, 6 x 4”, c. 1837, Bastion Place(?), listing of charges, payments by patrons (Jacob Bonesteel, William Britt, Andrew Britt, Nicolas Britt, George Eighmey, William H. Hendricks, Shultis mentioned) Ledger, tan leather- bound, 6 ¾ x 4”, labeled “Kingston Route”, c. 1866: misc. scattered notations Notebook with ledger entries, brown cardboard, 8 x 6 ¼”, missing back cover, names & charges throughout; page 1 has pasted illustration over entries, pp. 6-7 pasted paragraphs & poems, p. 6 from back, pasted prayer; p. 23 from back, pasted poems, pp. 34-35 from back, pasted story, “The Departed,” 1831-c.1842 Notebook, cat. no. 2004.001.0937/2036, 5 1/8 x 3 ¼”, inscr. back of front cover “March 13, 1885, Charles Hoyt’s book”(?) (only a few pages have entries; appear to be personal financial entries) Accounts – Shops & Stores – see file under Glass-making c. 1853 Adams, Arthur G., letter, 1973 Adirondack Mountains Advertisements Alderfer, Doug and Judy Alexander, William, 1726-1783 Altenau, H., see Saugerties, Population History files American Revolution Typescript by AE: list of Woodstock residents who served in armed forces during the Revolution & lived in Woodstock before and after the Revolution Photocopy, “Three Cemeteries of the Wynkoop Family,” N.Y. -

Alf Arvidsson Vladimir Propp's Fairy Tale Morphology and Game Studies
Computer games as fiction and social interaction A project sponsored by Vetenskapsrådet, 2003-2005 Institutionen för kultur och medier Umeå universitet Alf Arvidsson Vladimir Propp’s fairy tale morphology and game studies Working paper Vladimir Propp’s model of the structure of fairy tales has since its introduction to western scholarship during the 1950s, been regarded as one of the milestones in semiotic analysis and narratology, both for its inspiring function and taken for face value. It comes as no surprise that it also is suggested as a work of significance for game studies. I can agree on that; however, its meaning within the field of folklore studies and its range of appliability must be discussed. What is the content of Propp’s model? How has its basic ideas been further developed by later generations of folklorists? How can it be used? What claims can be based on it? What are its limits? My questions are trigged by the claims that sometimes are made for a universal applicability of Propp’s 31-function pattern. For instance, Arthur Asa Berger states in a recent introductory book, Video Games:A Popular Culture phenomenon, that “His thirty-one functions … are, it can be argued, at the heart of all narratives – not just the Russian folktales Propp analyzed in obtaining his list” (Berger 2002:34). I can agree with Berger that Propp’s model is worthy of attention in narrative studies. However, the claims of its universality go to far. It goes beyond the russian folktales, but halts somewhere after. The historical scientific context of Propp’s study is European folk tale studies, as they were in the 1920s with a history going back to the Grimm brothers showing the contemporary existence of an oral narrative genre with presumably ancient roots, within the popular classes. -

Adult Day Care, Supported Residential Care, and Assisted Living Healthcare Personnel Influenza Vaccination Coverage Report 2018-19 Influenza Season
STATE OF NEW HAMPSHIRE ADULT DAY CARE, SUPPORTED RESIDENTIAL CARE, AND ASSISTED LIVING HEALTHCARE PERSONNEL INFLUENZA VACCINATION COVERAGE REPORT 2018-19 INFLUENZA SEASON New Hampshire Department of Health and Human Services Division of Public Health Services State of New Hampshire Healthcare Personnel Influenza Vaccination ALF Report, 2018-19 Season LIST OF DATA TABLES Table 1. Influenza vaccination percentages for healthcare personnel by facility ....................... 15 Table 2. Influenza vaccination policies and consequences for healthcare personnel by assisted living facility .................................................................................................................................. 31 LIST OF FIGURES Figure 1. Statewide influenza vaccination percentages for healthcare personnel by influenza season ........................................................................................................................................... 14 Figure 2. Influenza vaccination percentages for healthcare personnel by facility ...................... 22 Figure 3. Influenza vaccination rate comparison by assisted living facility type .......................... 29 Figure 4. Influenza vaccination percentages for assisted living facility with and without vaccination policies ....................................................................................................................... 30 ABBREVIATIONS USED IN THIS DOCUMENT ALF Adult day care, supported residential care, and assisted living facilities CDC U.S. Centers -

Connecticut Wildlife Mar/Apr 2006
March/April 2006 PUBLISHED BY THE CONNECTICUT DEPARTMENT OF ENVIRONMENTAL PROTECTION BUREAU OF NATURAL RESOURCES ● WILDLIFE DIVISION ©PAUL J. FUSCO All Rights Reserved March/April 2006 Connecticut Wildlife 1 Volume 26, Number 2 ● March / April 2006 Connecticut From Wildlife Published bimonthly by State of Connecticut the irector Department of Environmental Protection D www.ct.gov/dep Gina McCarthy .................................................................. Commissioner David K. Leff ....................................................... Deputy Commissioner Edward C. Parker ........................... Chief, Bureau of Natural Resources “Oh, how I wish I had my camera.” Anybody who has encountered wildlife while afield has certainly repeated that lament on numerous Wildlife Division 79 Elm Street, Hartford, CT 06106-5127 (860-424-3011) occasions. My mental scrapbook is filled with awe inspiring images Dale May .................................................................................... Director that no one will ever see. My eyes have captured and my memory has Greg Chasko ................................................................ Assistant Director Mark Clavette ..................................................... Recreation Management enhanced these visions so they are clear, colorful, and spectacular – Laurie Fortin ............................................................... Wildlife Technician front page material for the finest magazines. From diving eagles to Elaine Hinsch ............................................................ -
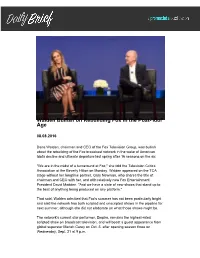
Walden Bullish on Rebuilding Fox in the Post-'Idol' Age
Walden Bullish on Rebuilding Fox in the Post-'Idol' Age 08.08.2016 Dana Walden, chairman and CEO of the Fox Television Group, was bullish about the rebuilding of the Fox broadcast network in the wake of American Idol's decline and ultimate departure last spring after 16 seasons on the air. "We are in the midst of a turnaround at Fox," she told the Television Critics Association at the Beverly Hilton on Monday. Walden appeared on the TCA stage without her longtime partner, Gary Newman, who shares the title of chairman and CEO with her, and with relatively new Fox Entertainment President David Madden. "And we have a slate of new shows that stand up to the best of anything being produced on any platform." That said, Walden admitted that Fox's summer has not been particularly bright and said the network has both scripted and unscripted shows in the pipeline for next summer, although she did not elaborate on what those shows might be. The network's current star performer, Empire, remains the highest-rated scripted show on broadcast television, and will boast a guest appearance from global superstar Mariah Carey on Oct. 5, after opening season three on Wednesday, Sept. 21 at 9 p.m. While Fox is taking some big swings on originals, it's also wrung some life out of reboots, particularly last winter's six-episode limited run of The X-Files and this year's upcoming dramas Lethal Weapon, 24: Legacy, which will premiere after the Super Bowl, and The Exorcist, for which Fox spent two nears negotiating through rights issues to be able to produce. -

Alf Hornborg
CORNUCOPIA OR ZERO-SUM GAME? THE EPISTEMOLOGY OF SusTAINABILITY* Alf Hornborg INTRODUCTION n the very first days of the new millennium, newspapers in Sweden-as O elsewhere-devoted some editorial space to assessing the state of the world. The leading daily Dagens Nyheter expressed puzzlement over a survey showing that a large percentage of Swedish youth were not particularly optimis tic about the future. Why this worry about global ecology, the editor asked, now that the pessimistic prophecies of the Club of Rome could be dismissed once and for all? Yet, the previous day, in the same newspaper, an environmental journal ist had observed that the state of the world environment is considerably worse than most people in the richer countries realize. The problem, he said, is that these people can choose to stay ignorant about the South's environment simply by switching television channels. Here were thus two very different messages on global ecology offered in the same newspaper. Similarly contradictory were its assessments of global inequality. On New Year's Eve, an editorial proclaimed that the Marxist notion that the affiuence of the rich is based on other people's impoverishment could be decisively dismissed. In the very same issue of Dagens Nyheter, however, an entry with the heading AlfHornborg Human Ecology Division Lund University Finngatan 16 223 62 Lund, Sweden ABSTRACT [email protected] This article contrasts two fundamentally world-economy. The article begins by sketching http ://www.humecol.lu.se/ different understandings of economic growth the history of these two perspectives in recent and "development" that lead to diametrically decades and reflecting on the ideological and * I would like to thank Rowman & Littlefield Publishers for permission to reprint opposed approaches to how to deal with epistemological contexts of their appearance this text, originally published as chapter 2 in my book The Power of the Machine: global ecological deterioration.