Thesis Croel
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phylogenetic Analysis of Anostracans (Branchiopoda: Anostraca) Inferred from Nuclear 18S Ribosomal DNA (18S Rdna) Sequences
MOLECULAR PHYLOGENETICS AND EVOLUTION Molecular Phylogenetics and Evolution 25 (2002) 535–544 www.academicpress.com Phylogenetic analysis of anostracans (Branchiopoda: Anostraca) inferred from nuclear 18S ribosomal DNA (18S rDNA) sequences Peter H.H. Weekers,a,* Gopal Murugan,a,1 Jacques R. Vanfleteren,a Denton Belk,b and Henri J. Dumonta a Department of Biology, Ghent University, Ledeganckstraat 35, B-9000 Ghent, Belgium b Biology Department, Our Lady of the Lake University of San Antonio, San Antonio, TX 78207, USA Received 20 February 2001; received in revised form 18 June 2002 Abstract The nuclear small subunit ribosomal DNA (18S rDNA) of 27 anostracans (Branchiopoda: Anostraca) belonging to 14 genera and eight out of nine traditionally recognized families has been sequenced and used for phylogenetic analysis. The 18S rDNA phylogeny shows that the anostracans are monophyletic. The taxa under examination form two clades of subordinal level and eight clades of family level. Two families the Polyartemiidae and Linderiellidae are suppressed and merged with the Chirocephalidae, of which together they form a subfamily. In contrast, the Parartemiinae are removed from the Branchipodidae, raised to family level (Parartemiidae) and cluster as a sister group to the Artemiidae in a clade defined here as the Artemiina (new suborder). A number of morphological traits support this new suborder. The Branchipodidae are separated into two families, the Branchipodidae and Ta- nymastigidae (new family). The relationship between Dendrocephalus and Thamnocephalus requires further study and needs the addition of Branchinella sequences to decide whether the Thamnocephalidae are monophyletic. Surprisingly, Polyartemiella hazeni and Polyartemia forcipata (‘‘Family’’ Polyartemiidae), with 17 and 19 thoracic segments and pairs of trunk limb as opposed to all other anostracans with only 11 pairs, do not cluster but are separated by Linderiella santarosae (‘‘Family’’ Linderiellidae), which has 11 pairs of trunk limbs. -

Federal Register/Vol. 68, No. 196/Thursday, October 9, 2003/Notices
Federal Register / Vol. 68, No. 196 / Thursday, October 9, 2003 / Notices 58355 Permit No. TE–050450 DEPARTMENT OF THE INTERIOR public review of this draft recovery plan. Applicant: Lisa Allen, Dana Point, Fish and Wildlife Service Recovery of endangered or threatened California. animals and plants is a primary goal of The permittee requests an amendment Re-Opening of the Comment Period for our endangered species program and the to take (harass by survey and collect and the Draft Recovery Plan for the Sierra Endangered Species Act (Act) (16 U.S.C. sacrifice) the Conservancy fairy shrimp Nevada Bighorn Sheep 1531 et seq.). Recovery means (Branchinecta conservatio), the AGENCY: U.S. Fish and Wildlife Service, improvement of the status of listed longhorn fairy shrimp (Branchinecta Interior. species to the point at which listing is longiantenna), the Riverside fairy no longer appropriate under the criteria ACTION: Notice of re-opening of public shrimp (Streptocephalus wootoni), the set out in section 4(a)(1) of the Act. comment period. San Diego fairy shrimp (Branchinecta Recovery plans describe actions sandiegonensis), and the vernal pool SUMMARY: We, the U.S. Fish and considered necessary for the tadpole shrimp (Lepidurus packardi) in Wildlife Service, announce a re-opening conservation of the species, establish conjunction with surveys throughout of the comment period for public review criteria for downlisting or delisting the range of each species in California of the Draft Recovery Plan for the Sierra listed species, and estimate time and for the purpose of enhancing their Nevada Bighorn Sheep (Ovis canadensis cost for implementing the measures survival. -

The Vernal Pool Landscape at the Nature Conservancy's Vina Plans
The Vernal Pool Landscape At The Nature Conservancy’s Vina Plans Preserve Presentation by Barbara Castro, California Department of Water Resources Text and photographs prepared by Rob Schlising, California State University, Chico (retired) Topics I Location, and history of, Vina Plains Preserve II The results of research done at VPP: a major resource III …and the U.S. Fish and Wildlife Service’s Vernal Pool Recovery Plan Red Bluff about 23 miles to the north Road in to old barn Original 1525 acres, with 4 fenced pastures Chico about 15 miles to the south Cemented volcanic mudflow underlies whole area and forms Northern Hardpan Vernal Pool bottoms paler green vegetation darker green vegetation reflects loamy soils reflects clayey soils barn Photo by Pauleen Broyles, winter 1983 Graduation of the first class of docents, spring 1983 The Nature Conservancy assembled a team of docents, spring 1983, that prepared in the field… Docents also prepared indoors. Graduation of the first class of docents, spring 1983 The Nature Conservancy hosted a dedication ceremony of the Vina Plains Preserve, at the old sheep-barn, on 16 April 1983 Mounts Lassen and Brokeoff showed that day 35 years ago 350 people attended, for a program by TNC—with a band, a catered chicken picnic lunch, and tours of the landscape by the docents. Landscape tours by the docents began for the pubic in 1984, starting from old logs located north of the sheep-barn. Over 1200 people visited VPP during the first several years of tours. The docent committee prepared a VPP handbook in 1984 (1994 revision shown) The Nature Conservancy has maintained ownership—and management—of the Preserve. -

VERNAL POOL TADPOLE SHRIMP Lepidurus Packardi
U.S. Fish & Wildlife Service Sacramento Fish & Wildlife Office Species Account VERNAL POOL TADPOLE SHRIMP Lepidurus packardi CLASSIFICATION: Endangered Federal Register 59-48136; September 19, 1994 http://ecos.fws.gov/docs/federal_register/fr2692.pdf On October 9, 2007, we published a 5-year review recommending that the species remain listed as endangered. CRITICAL HABITAT: Designated Originally designated in Federal Register 68:46683; August 6, 2003. The designation was revised in FR 70:46923; August 11, 2005. Species by unit designations were published in FR 71:7117 (PDF), February 10, 2006. RECOVERY PLAN: Final Recovery Plan for Vernal Pool Ecosystems of California and Southern Oregon, December 15, 2005. http://ecos.fws.gov/docs/recovery_plan/060614.pdf DESCRIPTION The vernal pool tadpole shrimp (Lepidurus packardi) is a small crustacean in the Triopsidae family. It has compound eyes, a large shield-like carapace (shell) that covers most of the body, and a pair of long cercopods (appendages) at the end of the last abdominal segment. Vernal pool tadpole shrimp adults reach a length of 2 inches in length. They have about 35 pairs of legs and two long cercopods. This species superficially resembles the rice field tadpole shrimp (Triops longicaudatus). Tadpole shrimp climb or scramble over objects, as well as plowing along or within bottom sediments. Their diet consists of organic debris and living organisms, such as fairy shrimp and other invertebrates. This animal inhabits vernal pools containing clear to highly turbid water, ranging in size from 54 square feet in the former Mather Air Force Base area of Sacramento County, to the 89-acre Olcott Lake at Jepson Prairie. -

Federal Register/Vol. 63, No. 151/Thursday, August
Federal Register / Vol. 63, No. 151 / Thursday, August 6, 1998 / Notices 42057 Dated: July 30, 1998. abandoned eggs and augmenting limited for the purpose of enhancing their Terry T. Terrell, populations in San Diego County, survival. Deputy Regional Director, Denver, Colorado. California, for the purpose of enhancing Permit No. 797266. [FR Doc. 98±21014 Filed 8±5±98; 8:45 am] its survival. Applicant: Dr. Douglas Alexander, Chico, California. BILLING CODE 4310±55±M Permit No. 844029. Applicant: Joseph S. Drennan, San The applicant requests an amendment Anselmo, California. to his permit to take (collect and DEPARTMENT OF THE INTERIOR The applicant requests a permit to sacrifice specimens, collect vernal pool take (harass by survey) the southwestern soil core samples) the Conservancy fairy Fish and Wildlife Service willow flycatcher (Empidonax trailli shrimp (Branchinecta conservatio) and extimus) in conjunction with surveys in the vernal pool tadpole shrimp Endangered Species Permit California, Nevada, and Arizona, for the (Lepidurus packardi) in Tehama and Applications purpose of enhancing its survival. Butte Counties, California, in Permit No. 844475. conjunction with food habits analysis AGENCY: Fish and Wildlife Service; Applicant: Shapiro and Associates, and scientific research for the purpose Interior. Inc., Portland, Oregon. of enhancing their survival. ACTION: Notice of receipt of permit The applicant requests a permit to DATES: Written comments on these applications. take (harass by survey) the Lost River permit applications -

Federal Register/Vol. 85, No. 38/Wednesday, February 26, 2020
11096 Federal Register / Vol. 85, No. 38 / Wednesday, February 26, 2020 / Notices ACTION: Notice. information, including any of the across three funding announcements. following subjects: (1) The necessity and Collection of performance measures is a SUMMARY: In compliance with the utility of the proposed information requirement of all TPP grant awards and requirement of the Paperwork collection for the proper performance of is included in the funding Reduction Act of 1995, the Office of the the agency’s functions; (2) the accuracy announcements. The measures include Secretary (OS), Department of Health of the estimated burden; (3) ways to dissemination, partners, training, and Human Services, is publishing the enhance the quality, utility, and clarity sustainability, reach, dosage, fidelity, following summary of a proposed of the information to be collected; and quality, Tier 1 supportive services collection for public comment. (4) the use of automated collection referrals, stakeholder engagement, and DATES: Comments on the ICR must be techniques or other forms of information Tier 2 Innovation project stage. To received on or before April 27, 2020. technology to minimize the information reflect the priorities of the new funding ADDRESSES: Submit your comments to collection burden. announcements, some of the measures [email protected] or by calling Title of the Collection: Teen and forms have been revised. The data (202) 795–7714. Pregnancy Prevention (TPP) collection will allow OPA to comply Performance Measures for FY2020. FOR FURTHER INFORMATION CONTACT: with federal accountability and Type of Collection: Revision. performance requirements, inform When submitting comments or OMB No. 0990–0438—OS-Office of requesting information, please include stakeholders of grantee progress in Population Affairs meeting TPP program goals, provide the document identifier 0990–0438– Abstract: The Office of Population OPA with metrics for monitoring 60D, and project title for reference, to Affairs (OPA), U.S. -

Federal Register/Vol. 63, No. 151/Thursday, August
Federal Register / Vol. 63, No. 151 / Thursday, August 6, 1998 / Notices 42057 Dated: July 30, 1998. abandoned eggs and augmenting limited for the purpose of enhancing their Terry T. Terrell, populations in San Diego County, survival. Deputy Regional Director, Denver, Colorado. California, for the purpose of enhancing Permit No. 797266. [FR Doc. 98±21014 Filed 8±5±98; 8:45 am] its survival. Applicant: Dr. Douglas Alexander, Chico, California. BILLING CODE 4310±55±M Permit No. 844029. Applicant: Joseph S. Drennan, San The applicant requests an amendment Anselmo, California. to his permit to take (collect and DEPARTMENT OF THE INTERIOR The applicant requests a permit to sacrifice specimens, collect vernal pool take (harass by survey) the southwestern soil core samples) the Conservancy fairy Fish and Wildlife Service willow flycatcher (Empidonax trailli shrimp (Branchinecta conservatio) and extimus) in conjunction with surveys in the vernal pool tadpole shrimp Endangered Species Permit California, Nevada, and Arizona, for the (Lepidurus packardi) in Tehama and Applications purpose of enhancing its survival. Butte Counties, California, in Permit No. 844475. conjunction with food habits analysis AGENCY: Fish and Wildlife Service; Applicant: Shapiro and Associates, and scientific research for the purpose Interior. Inc., Portland, Oregon. of enhancing their survival. ACTION: Notice of receipt of permit The applicant requests a permit to DATES: Written comments on these applications. take (harass by survey) the Lost River permit applications -

Crustacean Phylogeny…? Nauplius • First Larva Stage of Most “It Can Be Concluded That Crustacean Crustaceans
Bio 370 Crustacea Main arthropod clades (Regier et al 2010) Phylum Arthropoda http://blogs.discoverm • Trilobita agazine.com/loom/201 0/02/10/blind-cousins- Subphylum (or Class) Crustacea to-the-arthropod- • Chelicerata superstars/ Mostly aquatic, with calcified exoskeleton. • Mandibulata – Myriapoda (Chilopoda, Diplopoda) Head derived from acron plus next five segments- so primitively has 5 pairs of appendages: – Pancrustacea • Oligostraca (Ostracoda, Branchiura) -2 pair antennae • Altocrustacea - 1 pair of jaws – Vericrustacea - 2 pair of maxillae » (Branchiopoda, Decapoda) - usually a median (cyclopean) eye and – Miracrustacea one pair of compound eyes » Xenocarida (Remipedia, Cephalocarida) » Hexapoda Tagmosis of trunk varies in different taxa Crustacean phylogeny…? Nauplius • first larva stage of most “It can be concluded that crustacean crustaceans. phylogeny remains essentially unresolved. • three pairs of appendages • single median (naupliar) eye Conflict is rife, irrespective of whether one compares different morphological studies, molecular studies, or both.” Appendages: Jenner, 2010: Arthropod Structure & Development 39:143– -1st antennae 153 -2nd antennae - mandibles 1 Bio 370 Crustacea Crustacean taxa you should know Remipede habitat: a sea cave “blue hole” on Andros Island. Seven species are found in the Bahamas. Class Remipedia Class Malacostraca Class Branchiopoda “Peracarida”-marsupial crustacea Notostraca –tadpole shrimp Isopoda- isopods Anostraca-fairy shrimp Amphipoda- amphipods Cladocera- water fleas Mysidacea- mysids Conchostraca- clam shrimp “Eucarida” Class Maxillopoda Euphausiacea- krill Ostracoda- ostracods Decapoda- decapods- ten leggers Copepoda- copepods Branchiura- fish lice Penaeoidea- penaeid shrimp Cirripedia- barnacles Caridea- carid shrimp Astacidea- crayfish & lobsters Brachyura- true crabs Anomura- false crabs “Stomatopoda”– mantis shrimps Class Remipedia Remipides found only in sea caves in the Caribbean, the Canary Islands, and Western Australia (see pink below). -

Federal Register/Vol. 85, No. 135/Tuesday, July 14, 2020/Notices
42420 Federal Register / Vol. 85, No. 135 / Tuesday, July 14, 2020 / Notices authorized by 19 U.S.C. 58a–58c and 19 propagation or survival of endangered is issued that allows such activity. The U.S.C. 66, and set forth in 19 CFR 24.25. or threatened species under the ESA’s definition of ‘‘take’’ includes such CBP Form 400 is accessible at https:// Endangered Species Act. We invite the activities as pursuing, harassing, www.cbp.gov/newsroom/publications/ public and local, State, Tribal, and trapping, capturing, or collecting in forms, and is not being updated at this Federal agencies to comment on these addition to hunting, shooting, harming, time. applications. Before issuing any of the wounding, or killing. Estimated Number of Respondents: requested permits, we will take into A recovery permit issued by us under 1,443. consideration any information that we section 10(a)(1)(A) of the ESA Estimated Number of Annual receive during the public comment authorizes the permittee to conduct Responses per Respondent: 2. period. activities with endangered or threatened Estimated Number of Total Annual DATES: We must receive your written species for scientific purposes that Responses: 2,886. comments on or before August 13, 2020. Estimated Time per Response: 5 promote recovery or for enhancement of minutes (0.083 hours). ADDRESSES: Document availability and propagation or survival of the species. Estimated Total Annual Burden comment submission: Submit requests These activities often include such Hours: 240. for copies of the applications and prohibited actions as capture and related documents and submit any collection. Our regulations Dated: July 8, 2020. -
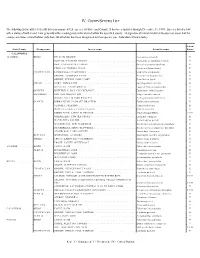
Iv. County/Species List
IV. COUNTY/SPECIES LIST The following list identifies federally listed or proposed U.S. species by State and County. It has been updated through December 31, 1999. Species listed below with a status of both E and T are generally either endangered or threatened within the specified county. Designation of critical habitat (CH) does not mean that the county constitutes critical habitat, only that critical habitat has been designated for that species (see Addendum A Instructions). Action/ State/County Group name Inverse name Scientific name Status CALIFORNIA ALAMEDA ......... BIRDS ....... PELICAN, BROWN ........................................ Pelicanus occidentalis E PLOVER, WESTERN SNOWY ................................ Charadrius alexandrinus nivosus T RAIL, CALIFORNIA CLAPPER ............................... Rallus longirostris obsoletus E TERN, CALIFORNIA LEAST ................................. Sterna antillarum browni E CRUSTACEAN . LINDERIELLA, CALIFORNIA ................................ Linderiella occidentalis E SHRIMP, LONGHORN FAIRY ................................ Branchinecta longiantenna E SHRIMP, VERNAL POOL FAIRY .............................. Branchinecta lynchi T FISHES ...... GOBY, TIDEWATER ...................................... Eucyclogobius newberryi E SPLITTAIL, SACRAMENTO ................................. Pogonichthys macrolepidotus T INSECTS ..... BUTTERFLY, BAY CHECKERSPOT ........................... Euphydryas editha bayensis T MAMMALS ... FOX, SAN JOAQUIN KIT .................................... Vulpes macrotis -

Crustacea: Branchiopoda: Notostraca) of the Espolla Temporary Pond in the Northeastern Iberian Peninsula
Hydrobiologia 486: 175–183, 2002. A.M. Maeda-Mart´ınez, B.V. Timms, D.C. Rogers, F.A. Abreu-Grobois & G. Murugan (eds), 175 Studies on Large Branchiopod Biology 4. © 2002 Kluwer Academic Publishers. Printed in the Netherlands. Population dynamics of Triops cancriformis (Crustacea: Branchiopoda: Notostraca) of the Espolla temporary pond in the northeastern Iberian peninsula Dani Boix, Jordi Sala & Ramon Moreno-Amich Institute of Aquatic Ecology and Department of Environmental Sciences, University of Girona Campus Montilivi. E-17071 Girona, Catalunya, Spain E-mail: [email protected] Key words: Triops cancriformis, sex ratio, fecundity, recruitment, hydroperiod duration Abstract The population dynamics of Triops cancriformis in Espolla temporary pond (NE Iberian peninsula) were studied during 1996 and 1997, which encompassed six flooded periods. Data were collected on each individual’s size, sex, and, if female, on number of eggs in the oostegopodes. Male-biased sex ratios were found only in the drying- out phase and variations in fecundity were strongly related to hydroperiod duration. Sex ratio variation during the drying-out phase can be attributed to female mortality because the very low recruitment observed does not support the hypothesis of an increase of males. Two hypotheses are advanced to account for female mortality: (1) differential reproductive effort, and (2) size selective predation by herons. This population is characterised by low values of maximum densities compared with other notostracan populations, and by higher densities in the spring–summer hydroperiods than in the winter ones. Introduction criformis is now a protected species. In spite of this protection and T. cancriformis’ local popularity (fest- In recent times, many notostracan and anostracan pop- ival, street, rock group and shops all bear the name of ulations in the Iberian peninsula have disappeared (Ar- this notostracan species), its population dynamics is mengol et al., 1975), because of the loss of many tem- poorly known. -

General Biological Resources Survey Report
Appendix B General Biological Resources Survey Report DRAFT GENERAL BIOLOGICAL RESOURCES SURVEY REPORT FOR THE E. & J. GALLO LIVINGSTON WATER INNOVATION AND ENERGY FACILITY PROJECT, MERCED COUNTY, CALIFORNIA (37°22’39.93”N, 120°48’37.66”W) P REPARED FOR: E & J Gallo Winery 18000 W River Road Livingston, CA 95334 Contact: Glenn Wright 209.394.5848 P REPARED BY: ICF International 75 E. Santa Clara Street, Suite 300 San José, CA 95113 Contact: Eric Christensen, Biologist 408.216.2819 email: [email protected] June 2012 ICF International. 2012. General Biological Resources Study for the E. & J. Gallo Winery Livingston Water Innovation and Energy Facility Project. Draft. June. (ICF 00265.12.) San José, CA. Prepared for E. & J. Gallo Winery, Livingston, CA. Contents List of Figures .......................................................................................................................................... ii List of Acronyms and Abbreviations ...................................................................................................... iii Page Chapter 1 Summary .................................................................................................................. 1‐1 Chapter 2 Introduction ............................................................................................................ 2‐1 Chapter 3 Methodology ............................................................................................................ 3‐1 Literature Reviewed ...........................................................................................................................