Crizanlizumab and Comparators for Adults with Sickle Cell Disease: a Systematic Review and Network Meta- Analysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DRUGS REQUIRING PRIOR AUTHORIZATION in the MEDICAL BENEFIT Page 1
Effective Date: 08/01/2021 DRUGS REQUIRING PRIOR AUTHORIZATION IN THE MEDICAL BENEFIT Page 1 Therapeutic Category Drug Class Trade Name Generic Name HCPCS Procedure Code HCPCS Procedure Code Description Anti-infectives Antiretrovirals, HIV CABENUVA cabotegravir-rilpivirine C9077 Injection, cabotegravir and rilpivirine, 2mg/3mg Antithrombotic Agents von Willebrand Factor-Directed Antibody CABLIVI caplacizumab-yhdp C9047 Injection, caplacizumab-yhdp, 1 mg Cardiology Antilipemic EVKEEZA evinacumab-dgnb C9079 Injection, evinacumab-dgnb, 5 mg Cardiology Hemostatic Agent BERINERT c1 esterase J0597 Injection, C1 esterase inhibitor (human), Berinert, 10 units Cardiology Hemostatic Agent CINRYZE c1 esterase J0598 Injection, C1 esterase inhibitor (human), Cinryze, 10 units Cardiology Hemostatic Agent FIRAZYR icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent HAEGARDA c1 esterase J0599 Injection, C1 esterase inhibitor (human), (Haegarda), 10 units Cardiology Hemostatic Agent ICATIBANT (generic) icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent KALBITOR ecallantide J1290 Injection, ecallantide, 1 mg Cardiology Hemostatic Agent RUCONEST c1 esterase J0596 Injection, C1 esterase inhibitor (recombinant), Ruconest, 10 units Injection, lanadelumab-flyo, 1 mg (code may be used for Medicare when drug administered under Cardiology Hemostatic Agent TAKHZYRO lanadelumab-flyo J0593 direct supervision of a physician, not for use when drug is self-administered) Cardiology Pulmonary Arterial Hypertension EPOPROSTENOL (generic) -

Novartis R&D and Investor Update
Novartis AG Investor Relations Novartis R&D and investor update November 5, 2018 Disclaimer This presentation contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995, that can generally be identified by words such as “potential,” “expected,” “will,” “planned,” “pipeline,” “outlook,” “agreement to acquire,” or similar expressions, or by express or implied discussions regarding potential marketing approvals, new indications or labeling for the investigational or approved products described in this presentation, or regarding potential future revenues from such products, or regarding the proposed acquisition of Endocyte, Inc. (Endocyte) by Novartis including the potential outcome and expected timing for completion of the proposed acquisition, and the potential impact on Novartis of the proposed acquisition, including express or implied discussions regarding potential future sales or earnings of Novartis, and any potential strategic benefits, synergies or opportunities expected as a result of the proposed acquisition. You should not place undue reliance on these statements. Such forward-looking statements are based on our current beliefs and expectations regarding future events, and are subject to significant known and unknown risks and uncertainties. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those set forth in the forward-looking statements. There can be no guarantee that the investigational or approved products described in this presentation will be submitted or approved for sale or for any additional indications or labeling in any market, or at any particular time. Nor can there be any guarantee that such products will be commercially successful in the future. -

Pharmacy Prior Authorization Grid ALTCS, and Pharmacy
Please Note: Refer to the other PA grids for applicable covered services that require PA. PA Grids: Medical, Behavioral Health, Pharmacy Prior Authorization Grid ALTCS, and Pharmacy. (Effective Date of Service 1/1/2021) Injectables that require Prior Authorization All chemotherapeutic drugs must be used for FDA-approved indications and/or in accordance with NCCN guidelines *Indicates prior authorization required if billed charges are greater than $400 PA Required HMO 13 HCPCS Short Description (BUCA- Code SNP) 90378 Respiratory Syncytial Virus Immune Globulin Yes C9036 Patisiran Yes C9047 Caplacizumab-yhdp Yes C9061 Teprotumumab-trbw Yes C9063 Eptinezumab-jjmr Yes C9131 Factor VIII antihemophilic factor pegylated-auci Yes C9132 Prothrombin Complex Concentrate (Human), Kcentra Yes C9133 Factor IX (Antihemophilic Factor, Recombinant), Rixibus Yes C9399 Mipomersen (Kynamro) Yes J0129 Abatacept Yes J0135 Adalimumab Yes J0178 Aflibercept Yes J0179 Brolucizumab-dbll, 1 mg Yes J0180 Agalsidase Beta Yes J0205 Alglucerase Yes J0215 Alefacept Yes J0220 Alglucosidase Alfa (Myozyme) Yes J0221 Alglucosidase Alfa (Lumizyme) Yes J0222 Patisiran, 0.1 mg Yes J0223 Givosiran 0.5 mg Yes J0256 Alpha 1-Proteinase Inhibitor Yes J0257 Alpha 1-Proteinase Inhibitor (Glassia) Yes J0275 Alprostadil Urethral Suppository Yes J0490 Belimumab Yes J0517 Benralizumab Yes J0567 Cerliponase alfa Yes J0570 Buprenorphine implant Yes J0584 Burosumab-twza 1 mg Yes J0585 Onabotulinumtoxina (Botox) Yes J0586 Abobotulinumtoxina (Dysport) Yes J0587 Rimabotulinumtoxinb (Myobloc) -

Crizanlizumab, Voxelotor, and L-Glutamine for Sickle Cell Disease: Effectiveness and Value
Crizanlizumab, Voxelotor, and L-Glutamine for Sickle Cell Disease: Effectiveness and Value Evidence Report Posted March 12, 2020 Updated February 9, 2021 Prepared for This evidence report was updated on February 9, 2021 with minor wording changes. ©Institute for Clinical and Economic Review, 2020 ICER Staff University of Calgary Pamela Bradt, MD, MPH Eldon Spackman, PhD Chief Scientific Officer Assistant Professor ICER Community Health Sciences & O'Brien Institute of Public Health University of Calgary Patricia G. Synnott, MALD, MS Director, Evidence Review ICER Rick Chapman, PhD, MS Director of Health Economics ICER Molly Beinfeld, MPH Research Lead, Evidence Synthesis ICER David M. Rind, MD, MSc Chief Medical Officer ICER Steven D. Pearson, MD, MSc President ICER The role of the University of Calgary is limited to the development of the cost-effectiveness model, and the resulting ICER reports do not necessarily represent the views of the University of Calgary. None of the above authors disclosed any conflicts of interest. How to cite this document: Bradt P, Spackman E, Synnott PG, Chapman R, Beinfeld M, Rind DM, Pearson SD. Crizanlizumab, Voxelotor, and L-Glutamine for Sickle Cell Disease: Effectiveness and Value. Institute for Clinical and Economic Review, January 23, 2020. https://icer.org/wp-content/uploads/2020/10/ICER_SCD_Evidence- Report_031220-FOR-PUBLICATION.pdf DATE OF PUBLICATION: March 12, 2020 Pamela Bradt served as the lead author for the report. Patricia Synnott led the systematic review and authorship of the comparative clinical effectiveness section in collaboration with Serina Herron-Smith and Avery McKenna. Patricia Synnott and Molly Beinfeld authored the chapter describing what ICER learned from patients (Chapter 2. -

Investor Presentation
Participants Company overview Pharmaceuticals Oncology Financial review Conclusion Appendix References Q1 2021 Results Investor presentation 1 Investor Relations │ Q1 2021 Results Participants Company overview Pharmaceuticals Oncology Financial review Conclusion Appendix References Disclaimer This presentation contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995, that can generally be identified by words such as “potential,” “expected,” “will,” “planned,” “pipeline,” “outlook,” or similar expressions, or by express or implied discussions regarding potential new products, potential new indications for existing products, potential product launches, or regarding potential future revenues from any such products; or regarding the impact of the COVID-19 pandemic on certain therapeutic areas including dermatology, ophthalmology, our breast cancer portfolio, some newly launched brands and the Sandoz retail and anti-infectives business, and on drug development operations; or regarding potential future, pending or announced transactions; regarding potential future sales or earnings of the Group or any of its divisions; or by discussions of strategy, plans, expectations or intentions; or regarding the Group’s liquidity or cash flow positions and its ability to meet its ongoing financial obligations and operational needs; or regarding our collaboration with Molecular Partners to develop, manufacture and commercialize potential medicines for the prevention and treatment of COVID- 19 and our joining of the industry-wide efforts to meet global demand for COVID-19 vaccines and therapeutics by leveraging our manufacturing capacity and capabilities to support the production of the Pfizer-BioNTech vaccine and to manufacture the mRNA and bulk drug product for the vaccine candidate CVnCoV from CureVac. -
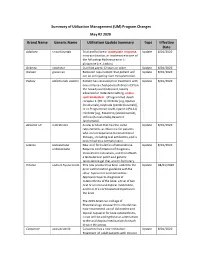
Summary of Utilization Management (UM) Program Changes May #2 2020
Summary of Utilization Management (UM) Program Changes May #2 2020 Brand Name Generic Name Utilization Update Summary Type Effective Date Adakveo crizanlizumab Trial and failure or inadequate response, Update 8/01/2020 contraindication, or intolerance to one of the following: Hydroxyurea or L- glutamine (i.e., Endari) Oxbryta voxelotor Clarified age to 12 years or older Update 8/01/2020 Givlaari givosiran Removed requirement that patient will Update 8/01/2020 not be anticipating liver transplantation. Padcev enfortumab vedotin Patient has received prior treatment with Update 8/01/2020 one immune checkpoint inhibitors (CPI) in the neoadjuvant/adjuvant, locally advanced or metastatic setting, unless contraindicated: i) Programmed death receptor-1 (PD-1) inhibitor [e.g.,Opdivo (nivolumab), Keytruda (pembrolizumab)], or ii) Programmed death-ligand 1 (PD-L1) inhibitor [e.g., Tecentriq (atezolizumab), Imfinzi (durvalumab), Bavencio (avelumab)]. Absorica LD isotretinoin A new product that has the same Update 8/01/2020 requirements as Absorica: for patients who are unresponsive to conventional therapy, including oral antibiotics, and is prescribed by a dermatologist. Jatenzo testosterone New oral formulation of testosterone. Update 8/01/2020 undecanoate Requires confirmation of diagnosis, testosterone lab values, and trial of both a testosterone patch and generic testosterone gel that are on formulary. Triluron sodium hyaluronate This new product has been added to the Update 08/01/2020 prior authorization guideline with the other hyaluronic acid derivatives. Approval requires diagnosis of osteoarthritis of the knee, a trial of two oral or an oral and topical medication, and trial of a corticosteroid injection in the knee. The 2019 American College of Rheumatology Osteoarthritis Guidelines now recommend use of duloxetine and topical capsaicin for knee osteoarthritis, so we will be adding these as alternatives to the oral/topical medications for each drug in this group. -

CDER Breakthrough Therapy Designation Approvals Data As of December 31, 2020 Total of 190 Approvals
CDER Breakthrough Therapy Designation Approvals Data as of December 31, 2020 Total of 190 Approvals Submission Application Type and Proprietary Approval Use Number Number Name Established Name Applicant Date Treatment of patients with previously BLA 125486 ORIGINAL-1 GAZYVA OBINUTUZUMAB GENENTECH INC 01-Nov-2013 untreated chronic lymphocytic leukemia in combination with chlorambucil Treatment of patients with mantle cell NDA 205552 ORIGINAL-1 IMBRUVICA IBRUTINIB PHARMACYCLICS LLC 13-Nov-2013 lymphoma (MCL) Treatment of chronic hepatitis C NDA 204671 ORIGINAL-1 SOVALDI SOFOSBUVIR GILEAD SCIENCES INC 06-Dec-2013 infection Treatment of cystic fibrosis patients age VERTEX PHARMACEUTICALS NDA 203188 SUPPLEMENT-4 KALYDECO IVACAFTOR 21-Feb-2014 6 years and older who have mutations INC in the CFTR gene Treatment of previously untreated NOVARTIS patients with chronic lymphocytic BLA 125326 SUPPLEMENT-60 ARZERRA OFATUMUMAB PHARMACEUTICALS 17-Apr-2014 leukemia (CLL) for whom fludarabine- CORPORATION based therapy is considered inappropriate Treatment of patients with anaplastic NOVARTIS lymphoma kinase (ALK)-positive NDA 205755 ORIGINAL-1 ZYKADIA CERITINIB 29-Apr-2014 PHARMACEUTICALS CORP metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib Treatment of relapsed chronic lymphocytic leukemia (CLL), in combination with rituximab, in patients NDA 206545 ORIGINAL-1 ZYDELIG IDELALISIB GILEAD SCIENCES INC 23-Jul-2014 for whom rituximab alone would be considered appropriate therapy due to other co-morbidities -

Pipeline Report August 2019
Pipeline Report August 2019 AcariaHealth Pipeline Report August 2019 Pipeline Report Recent Drug Approvals August 2019 The AcariaHealth Pipeline Report is a quarterly publication, provided by Envolve Pharmacy Solutions, created to help clients prepare for shifts in pharmacy benefit management. The report includes updates across multiple disease states including recent and anticipated drug approvals, key changes in the biosimilar agent landscape, and notes on recent and anticipated generic product launches. This report should only be used as a guide, as information may have changed from the time of publication. Recent Drug Approvals FDA AcariaHealth Cost Drug Name Manufacturer Indication(s) Drug Class Approval (AH) Access Comments (AWP Package Price) Date Status • One-time infusion. Zolgensma • Dramatic improvements in motor ability have been observed in infants who onasemnogene • Spinal muscular Neuromuscular $2.55 million / • Novartis 5/24/19 Limited Access received therapy. abeparvovec-xioi atrophy Type 1 agent one-time treatment • Approved with a Black Box Warning (BBW) re: acute serious liver injury. intravenous infusion • Spinraza is the only other FDA-approved agent. Piqray • For relapsed or refractory HR+, HER2-negative, PIK3CA-mutant advanced or alpelisib • Novartis • Breast cancer Oncology 5/24/19 AH has access $18,600 metastatic disease in both men and postmenopausal women. oral tablets Polivy • For use in combination with bendamustine and rituximab (BR). polatuzumab • Diffuse large B-cell $18,000 / cycle x 6 • Genentech Oncology 6/10/19 AH has access • Demonstrated significantly improved overall survival outcomes relative to vedotin-piiq lymphoma cycles total BR alone. intravenous infusion Xpovio • For use in combination with dexamethasone for patients who have received at • Karyopharm selinexor • Multiple myeloma Oncology 7/3/19 Limited access least four prior therapies. -

Horizon Scanning Status Report, Volume 2
PCORI Health Care Horizon Scanning System Volume 2, Issue 3 Horizon Scanning Status Report September 2020 Prepared for: Patient-Centered Outcomes Research Institute 1828 L St., NW, Suite 900 Washington, DC 20036 Contract No. MSA-HORIZSCAN-ECRI-ENG-2018.7.12 Prepared by: ECRI Institute 5200 Butler Pike Plymouth Meeting, PA 19462 Investigators: Randy Hulshizer, MA, MS Damian Carlson, MS Christian Cuevas, PhD Andrea Druga, PA-C Marcus Lynch, PhD, MBA Misha Mehta, MS Prital Patel, MPH Brian Wilkinson, MA Donna Beales, MLIS Jennifer De Lurio, MS Eloise DeHaan, BS Eileen Erinoff, MSLIS Cassia Hulshizer, AS Madison Kimball, MS Maria Middleton, MPH Diane Robertson, BA Melinda Rossi, BA Kelley Tipton, MPH Rosemary Walker, MLIS Andrew Furman, MD, MMM, FACEP Statement of Funding and Purpose This report incorporates data collected during implementation of the Patient-Centered Outcomes Research Institute (PCORI) Health Care Horizon Scanning System, operated by ECRI under contract to PCORI, Washington, DC (Contract No. MSA-HORIZSCAN-ECRI-ENG-2018.7.12). The findings and conclusions in this document are those of the authors, who are responsible for its content. No statement in this report should be construed as an official position of PCORI. An intervention that potentially meets inclusion criteria might not appear in this report simply because the Horizon Scanning System has not yet detected it or it does not yet meet inclusion criteria outlined in the PCORI Health Care Horizon Scanning System: Horizon Scanning Protocol and Operations Manual. Inclusion or absence of interventions in the horizon scanning reports will change over time as new information is collected; therefore, inclusion or absence should not be construed as either an endorsement or rejection of specific interventions. -

CDER Therapeutic Biologic Products List
CDER Therapeutic Biologic Products This list is intended to include all the Center for Drug Evaluation and Research (CDER) user fee billable therapeutic biological products and potencies approved under Section 351 of the Public Health Service Act. The Orange Book includes a section entitled "Drug Products with Approval under Section 505 of the Act Administered by CBER." Included on that list are several products that have been transferred to CDER which would be considered billable also. Program fees are assessed for each potency in which the approved (non-revoked, non-suspended) product is manufactured in final dosage form. When evaluating the specific strength or potency of a drug in final dosage form for purposes of assessing program fees for liquid parenteral biological products, CDER intends to take into consideration both the total amount of drug substance in mass or units of activity in a product and the concentration of drug substance (mass or units of activity per unit volume of product). Biologic products considered to have a different strength or potency in a final dosage form will be given separate entries in the Biologics List and assessed separate program fees. An auto-injector that has the same strength or potency as a prefilled syringe or vial will generally be assessed a separate prescription drug program fee. In certain circumstances, products which have been discontinued from marketing but are still licensed are not assessed program fees. Those products are identified on the CDER Discontinued Biologic Product List section. The potency information contained in this list is based on information in our database. -

OHA Recommendation
OFFICE OF THE DIRECTOR Kate Brown, Governor 500 Summer St NE E20 Salem OR 97301 Voice: 503-947-2340 Fax: 503-947-2341 OFFICIAL WEBSITE NOTICE www.Oregon.Gov/OHA Posting Date: June 9, 2020 www.health.oregon.gov RECOMMENDATIONS OF DRUG USE REVIEW / PHARMACY AND THERAPEUTICS COMMITTEE The Oregon Drug Use Review / Pharmacy and Therapeutics Committee met virtually on Thursday, June 4, 2020. The Committee considered in order of priority: the safety and efficacy of the drugs being considered, the ability of Oregonians to access effective prescription drugs that are appropriate for their clinical conditions and finally, substantial differences in costs of drugs within the same therapeutic class. Based upon the clinical information presented by staff iand all public comment offered,ii while considering the impact on special populations, the Committee makes the following recommendations for the Oregon Practitioner-Managed Prescription Drug Plan (PMPDP) or for any other preferred drug list established by the Oregon Health Authority: Practitioner-Managed Prescription Drug Plan (PMPDP) Recommendations: Acne Class Update with New Drug Evaluation (NDE) The Committee recommended making no changes to the PMPDP based on clinical evidence and to update the prior authorization (PA) criteria as proposed. After comparative cost consideration in executive session, the Committee recommended making Altreno™ (tretinoin) and Arazlo™ (tazarotene) non-preferred and unassigned benzoyl peroxide products preferred on the PMPDP, but subject to the acne PA criteria. DRUG CHANGE Altreno™ (tretinoin) Make non-preferred on the PMPDP Arazlo™ (tazarotene) Make non-preferred on the PMPDP unassigned benzoyl peroxide Make preferred on the PMPDP Antiepileptics Class Update with NDE The Committee recommended designating Xcopri® (cenobamate) as non-preferred on the PMPDP based on clinical evidence. -

Provider Administered Drugs – Site of Care – Oxford Clinical Policy
UnitedHealthcare® Oxford Clinical Policy Provider Administered Drugs – Site of Care Policy Number: PHARMACY 276.34 T2 Effective Date: October 1, 2021 Instructions for Use Table of Contents Page Related Policies ® Coverage Rationale ........................................ 2 • Actemra (Tocilizumab) Injection for Intravenous Infusion Documentation Requirements ....................... 3 ® • Adakveo (Crizanlizumab-Tmca) Definitions ....................................................... 3 • Alpha1-Proteinase Inhibitors Prior Authorization Requirements ................. 3 ™ • Amondys 45 (Casimersen) Applicable Codes ........................................... 4 ® • Benlysta (Belimumab) Clinical Evidence ............................................ 5 ® • Cimzia (Certolizumab Pegol) References ...................................................... 6 ® ® • Complement Inhibitors (Soliris & Ultomiris ) Policy History/Revision Information .............. 8 ® Instructions for Use ........................................ 8 • Crysvita (Burosumab) • Denosumab (Prolia® & Xgeva®) • Drug Coverage Guidelines • Entyvio® (Vedolizumab) • Evkeeza™ (Evinacumab-dgnb) • Exondys 51® (Eteplirsen) • Givlaari® (Givosiran) • Home Health Care • Ilaris® (Canakinumab) • Ilumya™ (Tildrakizumab-Asmn) • ™ ® ® ® Infliximab (Avsola , Inflectra , Remicade , Renflexis ) • Intravenous Enzyme Replacement Therapy (ERT) for Gaucher Disease • Long-Acting Injectable Antiretroviral Agents for HIV • Medical Therapies for Enzyme Deficiencies • Oncology Medication Clinical Coverage •