Accepted Version (PDF 557Kb)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Queensland Public Boat Ramps
Queensland public boat ramps Ramp Location Ramp Location Atherton shire Brisbane city (cont.) Tinaroo (Church Street) Tinaroo Falls Dam Shorncliffe (Jetty Street) Cabbage Tree Creek Boat Harbour—north bank Balonne shire Shorncliffe (Sinbad Street) Cabbage Tree Creek Boat Harbour—north bank St George (Bowen Street) Jack Taylor Weir Shorncliffe (Yundah Street) Cabbage Tree Creek Boat Harbour—north bank Banana shire Wynnum (Glenora Street) Wynnum Creek—north bank Baralaba Weir Dawson River Broadsound shire Callide Dam Biloela—Calvale Road (lower ramp) Carmilla Beach (Carmilla Creek Road) Carmilla Creek—south bank, mouth of creek Callide Dam Biloela—Calvale Road (upper ramp) Clairview Beach (Colonial Drive) Clairview Beach Moura Dawson River—8 km west of Moura St Lawrence (Howards Road– Waverley Creek) Bund Creek—north bank Lake Victoria Callide Creek Bundaberg city Theodore Dawson River Bundaberg (Kirby’s Wall) Burnett River—south bank (5 km east of Bundaberg) Beaudesert shire Bundaberg (Queen Street) Burnett River—north bank (downstream) Logan River (Henderson Street– Henderson Reserve) Logan Reserve Bundaberg (Queen Street) Burnett River—north bank (upstream) Biggenden shire Burdekin shire Paradise Dam–Main Dam 500 m upstream from visitors centre Barramundi Creek (Morris Creek Road) via Hodel Road Boonah shire Cromarty Creek (Boat Ramp Road) via Giru (off the Haughton River) Groper Creek settlement Maroon Dam HG Slatter Park (Hinkson Esplanade) downstream from jetty Moogerah Dam AG Muller Park Groper Creek settlement Bowen shire (Hinkson -

The Non-Human Reservoirs of Ross River Virus: a Systematic Review of the Evidence Eloise B
Stephenson et al. Parasites & Vectors (2018) 11:188 https://doi.org/10.1186/s13071-018-2733-8 REVIEW Open Access The non-human reservoirs of Ross River virus: a systematic review of the evidence Eloise B. Stephenson1*, Alison J. Peel1, Simon A. Reid2, Cassie C. Jansen3,4 and Hamish McCallum1 Abstract: Understanding the non-human reservoirs of zoonotic pathogens is critical for effective disease control, but identifying the relative contributions of the various reservoirs of multi-host pathogens is challenging. For Ross River virus (RRV), knowledge of the transmission dynamics, in particular the role of non-human species, is important. In Australia, RRV accounts for the highest number of human mosquito-borne virus infections. The long held dogma that marsupials are better reservoirs than placental mammals, which are better reservoirs than birds, deserves critical review. We present a review of 50 years of evidence on non-human reservoirs of RRV, which includes experimental infection studies, virus isolation studies and serosurveys. We find that whilst marsupials are competent reservoirs of RRV, there is potential for placental mammals and birds to contribute to transmission dynamics. However, the role of these animals as reservoirs of RRV remains unclear due to fragmented evidence and sampling bias. Future investigations of RRV reservoirs should focus on quantifying complex transmission dynamics across environments. Keywords: Amplifier, Experimental infection, Serology, Virus isolation, Host, Vector-borne disease, Arbovirus Background transmission dynamics among arboviruses has resulted in Vertebrate reservoir hosts multiple definitions for the key term “reservoir” [9]. Given Globally, most pathogens of medical and veterinary im- the diversity of virus-vector-vertebrate host interactions, portance can infect multiple host species [1]. -

Demographic Consequences of Superabundance in Krefft's River
i The comparative ecology of Krefft’s River Turtle Emydura krefftii in Tropical North Queensland. By Dane F. Trembath B.Sc. (Zoology) Applied Ecology Research Group University of Canberra ACT, 2601 Australia A thesis submitted in fulfilment of the requirements of the degree of Masters of Applied Science (Resource Management). August 2005. ii Abstract An ecological study was undertaken on four populations of Krefft’s River Turtle Emydura krefftii inhabiting the Townsville Area of Tropical North Queensland. Two sites were located in the Ross River, which runs through the urban areas of Townsville, and two sites were in rural areas at Alligator Creek and Stuart Creek (known as the Townsville Creeks). Earlier studies of the populations in Ross River had determined that the turtles existed at an exceptionally high density, that is, they were superabundant, and so the Townsville Creek sites were chosen as low abundance sites for comparison. The first aim of this study was to determine if there had been any demographic consequences caused by the abundance of turtle populations of the Ross River. Secondly, the project aimed to determine if the impoundments in the Ross River had affected the freshwater turtle fauna. Specifically this study aimed to determine if there were any difference between the growth, size at maturity, sexual dimorphism, size distribution, and diet of Emydura krefftii inhabiting two very different populations. A mark-recapture program estimated the turtle population sizes at between 490 and 5350 turtles per hectare. Most populations exhibited a predominant female sex-bias over the sampling period. Growth rates were rapid in juveniles but slowed once sexual maturity was attained; in males, growth basically stopped at maturity, but in females, growth continued post-maturity, although at a slower rate. -

Wetlands of the Townsville Area
A Final Report to the Townsville City Council WETLANDS OF THE TOWNSVILLE AREA ACTFR Report 96/28 25 November 1996 Prepared by G. Lukacs of the Australian Centre for Tropical Freshwater Research, James Cook University of North Queensland, Townsville Q 4811 Telephone (077 814262 Facsimile (077) 815589 Wetlands of the TCC LGA: Report No.96/28 TABLE OF CONTENTS 1. INTRODUCTION .................................................................................................................................. 1 1.1 Wetlands and the Community............................................................................................................. 1 1.2 The Wetlands of the Townsville Region ............................................................................................. 1 1.3 Values and Functions of Wetlands..................................................................................................... 3 2. METHODOLOGY ................................................................................................................................. 4 2.1 Scope .................................................................................................................................................. 4 2.2 Mapping ............................................................................................................................................. 4 2.3 Classification ..................................................................................................................................... 5 2.4 Sampling............................................................................................................................................ -

A Short History of Thuringowa
its 0#4, Wdkri Xdor# of fhurrngoraa Published by Thuringowa City Council P.O. Box 86, Thuringowa Central Queensland, 4817 Published October, 2000 Copyright The City of Thuringowa This book is copyright. Apart from any fair dealing for the purposes of private study, research, criticism or review, as permitted under the Copyright Act no part may be reproduced by any process without written permission. Inquiries should be addressed to the Publishers. All rights reserved. ISBN: 0 9577 305 3 5 kk THE CITY of Centenary of Federation i HURINGOWA Queensland This publication is a project initiated and funded by the City of Thuringowa This project is financially assisted by the Queensland Government, through the Queensland Community Assistance Program of the Centenary of Federation Queensland Cover photograph: Ted Gleeson crossing the Bohle. Gleeson Collection, Thuringowa Conienis Forward 5 Setting the Scene 7 Making the Land 8 The First People 10 People from the Sea 12 James Morrill 15 Farmers 17 Taking the Land 20 A Port for Thuringowa 21 Travellers 23 Miners 25 The Great Northern Railway 28 Growth of a Community 30 Closer Settlement 32 Towns 34 Sugar 36 New Industries 39 Empires 43 We can be our country 45 Federation 46 War in Europe 48 Depression 51 War in the North 55 The Americans Arrive 57 Prosperous Times 63 A great city 65 Bibliography 69 Index 74 Photograph Index 78 gOrtvard To celebrate our nations Centenary, and the various Thuringowan communities' contribution to our sense of nation, this book was commissioned. Two previous council publications, Thuringowa Past and Present and It Was a Different Town have been modest, yet tantalising introductions to facets of our past. -

WQ1181 - Ros! S River Basin
! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! Bohle River ! ! Stony Creek ! ! estuarine waters Town Common ! estuarine waters ! ! fresh waters ! ! ! ! ! Town Common ! ! ! estuarine waters ! ! ek ! e Cr ! ! ! r ! ! te a ! w ! ! ! *# e u ! l ek ! B re ! C ! ! ! ! ! ! ! ! y l a ! k e ! ! e r e Pallarenda fresh waters C H k ! ! ! ! ! e e ! ! r ! ! ! Deep C Cleveland Red Rock ! ! ! k ic l Pallarenda ! ! ! Bay A estuarine waters Ross Creek Bay ! ! Cape Cleveland ! estuarine waters ! ! fresh waters ! r ! ! e ! v ! ! i ! r R e ! v ! ! i R Rowes ! ! k c a e l l ! ! B Bay -
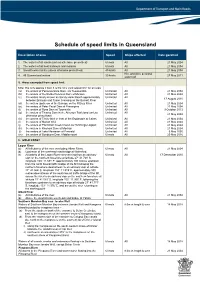
Schedule of Speed Limits in Queensland
Schedule of speed limits in Queensland Description of area Speed Ships affected Date gazetted 1. The waters of all canals (unless otherwise prescribed) 6 knots All 21 May 2004 2. The waters of all boat harbours and marinas 6 knots All 21 May 2004 3. Smooth water limits (unless otherwise prescribed) 40 knots All 21 May 2004 Hire and drive personal 4. All Queensland waters 30 knots 27 May 2011 watercraft 5. Areas exempted from speed limit Note: this only applies if item 3 is the only valid speed limit for an area (a) the waters of Perserverance Dam, via Toowoomba Unlimited All 21 May 2004 (b) the waters of the Bjelke Peterson Dam at Murgon Unlimited All 21 May 2004 (c) the waters locally known as Sandy Hook Reach approximately Unlimited All 17 August 2010 between Branyan and Tyson Crossing on the Burnett River (d) the waters upstream of the Barrage on the Fitzroy River Unlimited All 21 May 2004 (e) the waters of Peter Faust Dam at Proserpine Unlimited All 21 May 2004 (f) the waters of Ross Dam at Townsville Unlimited All 9 October 2013 (g) the waters of Tinaroo Dam in the Atherton Tableland (unless Unlimited All 21 May 2004 otherwise prescribed) (h) the waters of Trinity Inlet in front of the Esplanade at Cairns Unlimited All 21 May 2004 (i) the waters of Marian Weir Unlimited All 21 May 2004 (j) the waters of Plantation Creek known as Hutchings Lagoon Unlimited All 21 May 2004 (k) the waters in Kinchant Dam at Mackay Unlimited All 21 May 2004 (l) the waters of Lake Maraboon at Emerald Unlimited All 6 May 2005 (m) the waters of Bundoora Dam, Middlemount 6 knots All 20 May 2016 6. -

Status of Non-Native Freshwater Fishes in Tropical Northern
Journal & Proceedings of the Royal Society of New South Wales, Vol. 140, p. 63–78, 2007 ISSN 0035-9173/07/020063–16 $4.00/1 Status of Non-native Freshwater Fishes in Tropical Northern Queensland, Including Establishment Success, Rates of Spread, Range and Introduction Pathways alan charles webb Abstract: At least 20 non-native fishes have been reported from northern Queensland fresh waters, a 75% increase since 1994. Eleven of these species have established breeding populations and some are locally abundant and highly invasive, such as the tilapiine cichlids (Oreochromis mossambicus and Tilapia mariae) and the poeciliids (Gambusia holbrooki and Poecilia reticulata). Besides the continued introduction of non-native species, of great concern is the further spread of the tilapias, especially Oreochromis mossambicus and its hybrid form, and of another invasive, the three-spot gourami, Trichopterus trichogaster. Initial introductions are most probably releases of unwanted aquarium fish directly into open waters, or indirectly from ornamental ponds by flood waters. While natural dispersal is occurring, most of the range expansion of the tilapiine cichlids, particularly into impoundments in flood- prone areas, has been as a result of human translocation, and possibly the use of live bait by anglers. Keywords: Cichlidae, distribution patterns, Gambusia, Gourami, introduction pathways, invasive fishes, Oreochromis mossambicus, Poeciliidae, Tilapia INTRODUCTION native fishes in northern Queensland (McKay (1978, 1989, Arthington et al. 1984, Lear 1987), The history of non-native fishes, i.e., those orig- while McKay (1989) also referred to a previ- inating from overseas, introduced into northern ous, though unsuccessful, introduction of Jor- Queensland fresh waters has been well docu- danella sp. -

Mosquito-Borne Viruses and Non-Human Vertebrates in Australia: a Review
viruses Review Mosquito-Borne Viruses and Non-Human Vertebrates in Australia: A Review Oselyne T. W. Ong 1,2 , Eloise B. Skinner 3,4 , Brian J. Johnson 2 and Julie M. Old 5,* 1 Children’s Medical Research Institute, Westmead, NSW 2145, Australia; [email protected] 2 Mosquito Control Laboratory, QIMR Berghofer Medical Research Institute, Herston, QLD 4006, Australia; [email protected] 3 Environmental Futures Research Institute, Griffith University, Gold Coast, QLD 4222, Australia; [email protected] 4 Biology Department, Stanford University, Stanford, CA 94305, USA 5 School of Science, Western Sydney University, Hawkesbury, Locked bag 1797, Penrith, NSW 2751, Australia * Correspondence: [email protected] Abstract: Mosquito-borne viruses are well recognized as a global public health burden amongst humans, but the effects on non-human vertebrates is rarely reported. Australia, houses a number of endemic mosquito-borne viruses, such as Ross River virus, Barmah Forest virus, and Murray Valley encephalitis virus. In this review, we synthesize the current state of mosquito-borne viruses impacting non-human vertebrates in Australia, including diseases that could be introduced due to local mosquito distribution. Given the unique island biogeography of Australia and the endemism of vertebrate species (including macropods and monotremes), Australia is highly susceptible to foreign mosquito species becoming established, and mosquito-borne viruses becoming endemic alongside novel reservoirs. For each virus, we summarize the known geographic distribution, mosquito vectors, vertebrate hosts, clinical signs and treatments, and highlight the importance of including non-human vertebrates in the assessment of future disease outbreaks. The mosquito-borne viruses discussed can impact wildlife, livestock, and companion animals, causing significant changes to Australian Citation: Ong, O.T.W.; Skinner, E.B.; ecology and economy. -

Great Barrier Reef
PAPUA 145°E 150°E GULF OF PAPUA Dyke NEW GUINEA O Ackland W Bay GREAT BARRIER REEF 200 E Daru N S General Reference Map T Talbot Islands Anchor Cay A Collingwood Lagoon Reef N Bay Saibai Port Moresby L Reefs E G Island Y N E Portlock Reefs R A Torres Murray Islands Warrior 10°S Moa Boot Reef 10°S Badu Island Island 200 Eastern Fields (Refer Legend below) Ashmore Reef Strait 2000 Thursday 200 Island 10°40’55"S 145°00’04"E WORLD HERITAGE AREA AND REGION BOUNDARY ait Newcastle Bay Endeavour Str GREAT BARRIER REEF WORLD HERITAGE AREA Bamaga (Extends from the low water mark of the mainland and includes all islands, internal waters of Queensland and Seas and Submerged Lands Orford Bay Act exclusions.) Total area approximately 348 000 sq km FAR NORTHERN Raine Island MANAGEMENT AREA GREAT BARRIER REEF REGION CAPE Great Detached (Extends from the low water mark of the mainland but excludes lburne Bay he Reef Queensland-owned islands, internal waters of Queensland and Seas S and Submerged Lands Act exclusions.) Total area approximately 346 000 sq km ple Bay em Wenlock T GREAT BARRIER REEF MARINE PARK (Excludes Queensland-owned islands, internal waters of Queensland River G and Seas and Submerged Lands Act exclusions.) Lockhart 4000 Total area approximately 344 400 sq km Weipa Lloyd Bay River R GREAT BARRIER REEF MARINE PARK 12°59’55"S MANAGEMENT AREA E 145°00’04"E CORAL SEA YORK GREAT BARRIER REEF PROVINCE Aurukun River A (As defined by W.G.H. -

Submission to Queensland Floods Commission of Enquiry
1 Page 1 Submission to Queensland Floods Commission of Enquiry The 2004 Crime and Misconduct Commissions investigation into Brisbane River Flood Levels is an example of how a risk is identified by engineering modelling and then official complaints received and investigated and in the follow up works and policies that could have mitigated or reduced the risks did not occur. Given perfect 20 – 20 hindsight the CMC and the Brisbane City Council got it wrong and Sinclair Knight Mertz got it right. This submission is not focused on Brisbane but flood management of the Bohle River, the Ross River and operation of the Ross Dam in Townsville City and modern surveying techniques as they relate to survey control and measurement of Australian Height Datum. It is my hope that these issues fall within the Terms of Reference of the Commission of Enquiry. BOHLE RIVER In 1993 the Thuringowa City Council commissioned a Bohle River Flood Mitigation Study. This study modelled the Bohle River and established AR&R Q50 development lines using the 1987 AR&R Rainfall Data. The report recommended channel clearing at an estimated cost of $88,000. This work was not carried out. In 2000 the Thuringowa City Council accepted the Bohle River Floodplain Management Study and in this instance I was elected to be a residents representative on the steering committee. I witnessed the Mike 11 and Mike 21 modelling as presented by the consultant’s representative and was verbally informed of the impacts of a Probable Maximum Flood (PMF). The draft report when released showed river long sections of several flooding scenarios including that of a PMF. -

Surface Water Network Review Final Report
Surface Water Network Review Final Report 16 July 2018 This publication has been compiled by Operations Support - Water, Department of Natural Resources, Mines and Energy. © State of Queensland, 2018 The Queensland Government supports and encourages the dissemination and exchange of its information. The copyright in this publication is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) licence. Under this licence you are free, without having to seek our permission, to use this publication in accordance with the licence terms. You must keep intact the copyright notice and attribute the State of Queensland as the source of the publication. Note: Some content in this publication may have different licence terms as indicated. For more information on this licence, visit https://creativecommons.org/licenses/by/4.0/. The information contained herein is subject to change without notice. The Queensland Government shall not be liable for technical or other errors or omissions contained herein. The reader/user accepts all risks and responsibility for losses, damages, costs and other consequences resulting directly or indirectly from using this information. Interpreter statement: The Queensland Government is committed to providing accessible services to Queenslanders from all culturally and linguistically diverse backgrounds. If you have difficulty in understanding this document, you can contact us within Australia on 13QGOV (13 74 68) and we will arrange an interpreter to effectively communicate the report to you. Surface