PELLAGRA and Its Prevention and Control in Major Emergencies ©World Health Organization, 2000
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Familial Pellagra-Like Skin Rash with Neurological Manifestations
Arch Dis Child: first published as 10.1136/adc.56.2.146 on 1 February 1981. Downloaded from 146 Freundlich, Statter, and Yatziv Familial pellagra-like skin rash with neurological manifestations E FREUNDLICH, M STATTER, AND S YATZIV Department ofPaediatrics, Government Hospital, Nahariya, Israel, and Paediatric Research Unit and Department ofPaediatrics, Hadassah University Hospital, Jerusalem, Israel development was normal, although the skin rash SUMMARY A 14-year-old boy of Arabic origin was noted several times. On one occasion a skin presented with a pellagra-like rash and neurological biopsy was performed and reported to be compatible manifestations including ataxia, dysarthria, nystag- with pellagra. A striking improvement was again mus, and coma. There was a striking response to oral noticed after nicotinamide administration. nicotinamide. The laboratory findings were not The last admission was at age 14 years in late typical of Hartnup disease: aminoaciduria and November (as were all previous admissions). At indicanuria were absent and there was no evidence this time, he presented with mild confusion, diplopia, of tryptophan malabsorption. Tryptophan loading dysarthria, ataxia, and a pellagroid skin rash. His did not induce tryptophanuria nor did it increase general condition deteriorated during the next few excretion of xanthurenic or kynurenic acids. These days; he became unable to walk or stand, and both findings support the possibility of a block in trypto- horizontal and vertical nystagmus were observed. phan degradation. The family history suggests a He became apathetic and entered into deep coma genetically-determined disorder. 4 days after admission. The electroencephalogram showed a markedly abnormal tracing with short We report a patient with familial pellagra-like skin bursts of high voltage 2 5 per second activity with manifestations and laboratory rash, with neurological superimposed sharp waves, mainly over the posterior copyright. -

THE PATHOLOGIC PHYSIOLOGY of PELLAGRA: V. the Circulating Blood Volume
THE PATHOLOGIC PHYSIOLOGY OF PELLAGRA: V. The Circulating Blood Volume Roy H. Turner J Clin Invest. 1931;10(1):111-120. https://doi.org/10.1172/JCI100332. Find the latest version: https://jci.me/100332/pdf THE PATHOLOGIC PHYSIOLOGY OF PELLAGRA V. THE CIRCULATING BLOOD VOLUME By ROY H. TURNER (From the Department of Medicine, Tulane University of Louisiana School of Medicine, and the Medical Services of the Charity Hospital, New Orleans) (Received for publication October 27, 1930) Determination of circulating blood volume was undertaken as a part of a study of the disturbed physiology in pellagra, chiefly for the purpose of finding out whether shrinkage of plasma volume existed in patients who were suffering from a disease frequently characterized by severe diarrhea. Such a shrinkage would be of great importance of itself, and would obviously have a bearing upon the interpretation of the composition of the plasma determined at the same time. The existence of anemia can hardly be established nor its severity estimated -so long as we are in ignorance- of the plasma volume. It was also considered possible that the magnitude of the circulating blood volume might be correlated with certain of the features of the skin lesions, such as the degree of exudation of serum. I have used the dye method of Keith, Rowntree and Geraghty (1) modified as follows: A 3 per cent aqueous solution of brilliant vital red (National Analine Company) was made up 'the afternoon before use, and sterilized at 100°C. for 8 minutes. With a sterile calibrated pipette the quantity of this solution for each patient was placed in a sterile 50 cc. -

Folic Acid, Pyridoxine, and Cyanocobalamin Combination
ORIGINAL INVESTIGATION Folic Acid, Pyridoxine, and Cyanocobalamin Combination Treatment and Age-Related Macular Degeneration in Women The Women’s Antioxidant and Folic Acid Cardiovascular Study William G. Christen, ScD; Robert J. Glynn, ScD; Emily Y. Chew, MD; Christine M. Albert, MD; JoAnn E. Manson, MD Background: Observational epidemiologic studies indi- and visually significant AMD, defined as confirmed in- cate a direct association between homocysteine concentra- cident AMD with visual acuity of 20/30 or worse attrib- tion in the blood and the risk of age-related macular degen- utable to this condition. eration (AMD), but randomized trial data to examine the effect of therapy to lower homocysteine levels in AMD are Results:Afteranaverageof7.3yearsoftreatmentandfollow- lacking. Our objective was to examine the incidence of AMD up, there were 55 cases of AMD in the combination treat- in a trial of combined folic acid, pyridoxine hydrochloride ment group and 82 in the placebo group (relative risk, 0.66; (vitamin B6), and cyanocobalamin (vitamin B12) therapy. 95% confidence interval, 0.47-0.93 [P=.02]). For visually significant AMD, there were 26 cases in the combination Methods: We conducted a randomized, double-blind, treatment group and 44 in the placebo group (relative risk, placebo-controlled trial including 5442 female health care 0.59; 95% confidence interval, 0.36-0.95 [P=.03]). professionals 40 years or older with preexisting cardio- vascular disease or 3 or more cardiovascular disease risk Conclusions: These randomized trial data from a large factors. A total of 5205 of these women did not have a cohort of women at high risk of cardiovascular disease diagnosis of AMD at baseline and were included in this indicate that daily supplementation with folic acid, pyri- analysis. -

R Graphics Output
Dexamethasone sodium phosphate ( 0.339 ) Melengestrol acetate ( 0.282 ) 17beta−Trenbolone ( 0.252 ) 17alpha−Estradiol ( 0.24 ) 17alpha−Hydroxyprogesterone ( 0.238 ) Triamcinolone ( 0.233 ) Zearalenone ( 0.216 ) CP−634384 ( 0.21 ) 17alpha−Ethinylestradiol ( 0.203 ) Raloxifene hydrochloride ( 0.203 ) Volinanserin ( 0.2 ) Tiratricol ( 0.197 ) trans−Retinoic acid ( 0.192 ) Chlorpromazine hydrochloride ( 0.191 ) PharmaGSID_47315 ( 0.185 ) Apigenin ( 0.183 ) Diethylstilbestrol ( 0.178 ) 4−Dodecylphenol ( 0.161 ) 2,2',6,6'−Tetrachlorobisphenol A ( 0.156 ) o,p'−DDD ( 0.155 ) Progesterone ( 0.152 ) 4−Hydroxytamoxifen ( 0.151 ) SSR150106 ( 0.149 ) Equilin ( 0.3 ) 3,5,3'−Triiodothyronine ( 0.256 ) 17−Methyltestosterone ( 0.242 ) 17beta−Estradiol ( 0.24 ) 5alpha−Dihydrotestosterone ( 0.235 ) Mifepristone ( 0.218 ) Norethindrone ( 0.214 ) Spironolactone ( 0.204 ) Farglitazar ( 0.203 ) Testosterone propionate ( 0.202 ) meso−Hexestrol ( 0.199 ) Mestranol ( 0.196 ) Estriol ( 0.191 ) 2,2',4,4'−Tetrahydroxybenzophenone ( 0.185 ) 3,3,5,5−Tetraiodothyroacetic acid ( 0.183 ) Norgestrel ( 0.181 ) Cyproterone acetate ( 0.164 ) GSK232420A ( 0.161 ) N−Dodecanoyl−N−methylglycine ( 0.155 ) Pentachloroanisole ( 0.154 ) HPTE ( 0.151 ) Biochanin A ( 0.15 ) Dehydroepiandrosterone ( 0.149 ) PharmaCode_333941 ( 0.148 ) Prednisone ( 0.146 ) Nordihydroguaiaretic acid ( 0.145 ) p,p'−DDD ( 0.144 ) Diphenhydramine hydrochloride ( 0.142 ) Forskolin ( 0.141 ) Perfluorooctanoic acid ( 0.14 ) Oleyl sarcosine ( 0.139 ) Cyclohexylphenylketone ( 0.138 ) Pirinixic acid ( 0.137 ) -

Scurvy Due to Selective Diet in a Seemingly Healthy 4-Year-Old Boy Andrew Nastro, MD,A,G,H Natalie Rosenwasser, MD,A,B Steven P
Scurvy Due to Selective Diet in a Seemingly Healthy 4-Year-Old Boy Andrew Nastro, MD,a,g,h Natalie Rosenwasser, MD,a,b Steven P. Daniels, MD,c Jessie Magnani, MD,a,d Yoshimi Endo, MD,e Elisa Hampton, MD,a Nancy Pan, MD,a,b Arzu Kovanlikaya, MDf Scurvy is a rare disease in developed nations. In the field of pediatrics, it abstract primarily is seen in children with developmental and behavioral issues, fDivision of Pediatric Radiology and aDepartments of malabsorptive processes, or diseases involving dysphagia. We present the Pediatrics and cRadiology, NewYork-Presbyterian/Weill Cornell Medical Center, New York, New York; bDivision of case of an otherwise developmentally appropriate 4-year-old boy who Pediatric Rheumatology and eDepartment of Radiology, developed scurvy after gradual self-restriction of his diet. He initially Hospital for Special Surgery, New York, New York; and d presented with a limp and a rash and was subsequently found to have anemia Division of Neonatal-Perinatal Medicine, University of Michigan, Ann Arbor, Michigan gDepartment of Pediatrics, and hematuria. A serum vitamin C level was undetectable, and after review of NYU School of Medicine, New York, New York hDepartment of the MRI of his lower extremities, the clinical findings supported a diagnosis of Pediatrics, Bellevue Hospital Center, New York, New York scurvy. Although scurvy is rare in developed nations, this diagnosis should be Dr Nastro helped conceptualize the case report, considered in a patient with the clinical constellation of lower-extremity pain contributed to writing the introduction, initial presentation, hospital course, and discussion, and or arthralgias, a nonblanching rash, easy bleeding or bruising, fatigue, and developed the laboratory tables while he was anemia. -

Kwashiokorl. Description of Kwashiorkor Kwashiorkor Is a Life-Threatening and Debilitating Form of Malnutrition. It's Caused B
Name ;OLAWUYI MUQTADIR OREDOLAPO COUSE AFE 101 DEPARTMENT ;ACCOUNTING KWASHIOKORl. Description of kwashiorkor kwashiorkor is a life-threatening and debilitating form of malnutrition. It’s caused by a lack of protein in your diet. Kwashiorkor is also commonly seen in low- and lower-middle-income regions facing famine. Signs and symptoms of kwashiorkor The symptoms of kwashiorkor include; • damaged immune system, which can lead to more frequent and severe infections • irritability. • flaky rash • shock. • change in skin and hair colour (to a rust colour) and texture • fatigue • diarrhea • loss of muscle mass • failure to grow or gain weight • edema (swelling) of the ankles, feet, and belly SUSCEPTIBLES If kwashiorkor is suspected, your doctor will first examine you to check for an enlarged liver (hepatomegaly) and swelling. Next, blood and urine tests may be ordered to measure the level of protein and sugar in your blood. Other tests may be performed on your blood and urine to measure signs of malnutrition and lack of protein. These tests may look for muscle breakdown and assess kidney function, overall health, and growth. These tests include: arterial blood gas, blood urea nitrogen , urinalysis e.t.c. TREATMENT Kwashiorkor can be corrected by eating more protein and more calories overall, especially if treatment is started early. You may first be given more calories in the form of carbohydrates, sugars, and fats. Once these calories provide energy, you will be given foods with proteins. Foods must be introduced and calories should be increased slowly because you have been without proper nutrition for a long period. Your body may need to adjust to the increased intake. -

Guidelines on Food Fortification with Micronutrients
GUIDELINES ON FOOD FORTIFICATION FORTIFICATION FOOD ON GUIDELINES Interest in micronutrient malnutrition has increased greatly over the last few MICRONUTRIENTS WITH years. One of the main reasons is the realization that micronutrient malnutrition contributes substantially to the global burden of disease. Furthermore, although micronutrient malnutrition is more frequent and severe in the developing world and among disadvantaged populations, it also represents a public health problem in some industrialized countries. Measures to correct micronutrient deficiencies aim at ensuring consumption of a balanced diet that is adequate in every nutrient. Unfortunately, this is far from being achieved everywhere since it requires universal access to adequate food and appropriate dietary habits. Food fortification has the dual advantage of being able to deliver nutrients to large segments of the population without requiring radical changes in food consumption patterns. Drawing on several recent high quality publications and programme experience on the subject, information on food fortification has been critically analysed and then translated into scientifically sound guidelines for application in the field. The main purpose of these guidelines is to assist countries in the design and implementation of appropriate food fortification programmes. They are intended to be a resource for governments and agencies that are currently implementing or considering food fortification, and a source of information for scientists, technologists and the food industry. The guidelines are written from a nutrition and public health perspective, to provide practical guidance on how food fortification should be implemented, monitored and evaluated. They are primarily intended for nutrition-related public health programme managers, but should also be useful to all those working to control micronutrient malnutrition, including the food industry. -

Involvement of the Eye in Protein Malnutrition * -303
Bull. Org. mond. Sante 1958, 19, 303-314 Bull. Wld Hith Org. INVOLVEMENT OF THE EYE IN PROTEIN MALNUTRITION * D. S. McLAREN, M.D., Ph.D., D.T.M. & H. Medical Research Officer, East African Institute for Medical Research, Mwanza, Tanganyika Formerly at the MRC Human Nutrition Research Unit, National Institute for Medical Research, London SYNOPSIS An extensive review of the literature on protein malnutrition, with special reference to the frequency of involvement of the eyes, has been made by the author. Consideration of accounts from all parts of the world and in many different languages, including early as well as more recent descriptions of the syndrome, indicates that this important complication has not received sufficient attention hitherto. The evidence available suggests that it is nearly always an accompanying deficiency of vitamin A that is responsible. Less commonly reported-and producing less severe effects-is deficiency of the B-complex vitamins, and there is no clear evidence to date that protein deficiency itself damages the eyes in these cases. The ways in which protein lack might interfere with various aspects of vitamin-A metabolism are discussed, but it is pointed out that their actual significance in human disease is not yet known. A low dietary intake of vitamin A is regarded by the author as being the prime factor in the causation of eye complications, and attention is drawn to the necessity to correct this as part of any prophylactic or therapeutic programme aimed primarily at combat- ing protein malnutrition. The syndrome known by such various names as kwashiorkor, nutritional oedema syndrome, sindrome pluricarencial, and many others (Trowell, Davies & Dean, 1954), and characterized chiefly by a dietary deficiency ofprotein, has been reported as occurring amongst most of the malnourished communities of the world. -

These Highlights Do Not Include All the Information Needed to Use M.V.I. Pediatric® Safely and Effectively
M.V.I. PEDIATRIC- ascorbic acid, retinol, ergocalciferol, thiamine hydrochloride, riboflavin 5- phosphate sodium, pyridoxine hydrochloride, niacinamide, dexpanthenol, .alpha.-tocopherol acetate, dl-, biotin, folic acid, cyanocobalamin, and phytonadione injection, powder, lyophilized, for solution Hospira, Inc. ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use M.V.I. Pediatric® safely and effectively. See full prescribing information for M.V.I. Pediatric. M.V.I. Pediatric (multiple vitamins for injection), for intravenous use Initial U.S. Approval: 1983 RECENT MAJOR CHANGES Dosage And Administration, Dosage Information (2.2) 2/2019 INDICATIONS AND USAGE M.V.I. Pediatric is a combination of vitamins indicated for the prevention of vitamin deficiency in pediatric patients up to 11 years of age receiving parenteral nutrition (1) DOSAGE AND ADMINISTRATION M.V.I. Pediatric is a combination product that contains the following vitamins: ascorbic acid, vitamin A, vitamin D, thiamine, riboflavin, pyridoxine, niacinamide, dexpanthenol, vitamin E, vitamin K, folic acid, biotin, and vitamin B12 (2.1) Supplied as a single-dose vial of lyophilized powder for reconstitution intended for administration by intravenous infusion after dilution. (2.1) Recommended daily dosage is based on patient's actual weight (2.2) Less than 1 kg: The daily dose is 1.5 mL 1 kg to 3 kg: The daily dose is 3.25 mL 3 kg or more: The daily dose is 5 mL One daily dose of the reconstituted solution (1.5 mL, 3.25 mL or 5 mL) is then added directly to the intravenous fluid (2.2,2.3) See Full Prescribing Information for reconstitution instructions (2.3) Monitor blood vitamin concentrations (2.4) See Full Prescribing Information for drug incompatibilities (2.5) DOSAGE FORMS AND STRENGTHS M.V.I. -

Becosules Junior
For the use of a Registered Medical Practitioner or a Hospital or a Laboratory only Multivitamin with Vitamin A and Vitamin D3 Liquid BECOSULES JUNIOR 1. NAME OF THE MEDICINAL PRODUCT BECOSULES JUNIOR 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 5 ml (1 teaspoonful) contains: Vitamin A Concentrate Oil I.P. (as Palmitate) 2500 IU Cholecalciferol I.P. 200 IU Thiamine Hydrochloride I.P. 2 mg Riboflavin Sodium Phosphate I.P. 2.54 mg Pyridoxine Hydrochloride I.P. 1 mg Niacinamide I.P. 20 mg D-Panthenol I.P. 5 mg Ascorbic Acid I.P. 50 mg For Pediatric Use For a full list of excipients, see section 6.1. 3. PHARMACOLOGICAL FORM Liquid Trademark Owner: Pfizer Products Inc. USA; Licensed User: Pfizer Limited, India BECOSULES JUNIOR Page 1 of 9 LPDBECJR122017 4. CLINICAL PARTICULARS 4.1 Indications Becosules Junior is indicated in the treatment of patients with deficiencies of, or increased requirement for vitamins A, B complex, C and D. Such patients and conditions include: • Decreased intake because of restricted or unbalanced diet as in anorexia, diabetes mellitus and obesity, and insufficient sunlight exposure.1 • Reduced availability during treatment with antimicrobials which alter normal intestinal flora, and anticonvulsants and glucocorticoids which alter vitamin D metabolism1, in prolonged diarrhea and in chronic gastrointestinal disorders. • Increased requirements due to increased metabolic rate as in fever and tissue wasting, e.g. febrile illness, acute or chronic infections, surgery, burns and fractures. • Stomatitis, glossitis, cheilosis, paraesthesias, neuralgia and dermatitis. 4.2 Posology and Method of Administration For children from 1-3 years - 1.25 ml, 4-9 years - 2.5 ml; and 10-13 years - 5 ml or as directed by physician. -

B-COMPLEX FORTE with VITAMIN C CAPSULES BECOSULES Capsules
For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory. B-COMPLEX FORTE WITH VITAMIN C CAPSULES BECOSULES Capsules 1. NAME OF THE MEDICINAL PRODUCT BECOSULES 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains: Thiamine Mononitrate I.P. 10 mg Riboflavin I.P. 10 mg Pyridoxine Hydrochloride I.P. 3 mg Vitamin B12 I.P. ( as STABLETS 1:100) 15 mcg Niacinamide I.P. 100 mg Calcium Pantothenate I.P. 50 mg Folic Acid I.P. 1.5 mg Biotin U.S.P. 100 mcg Ascorbic Acid I.P. (as coated) 150 mg Appropriate overages added For Therapeutic Use For a full list of excipients, see section 6.1. All strengths/presentations mentioned in this document might not be available in the market. 3. PHARMACOLOGICAL FORM Capsules 4. CLINICAL PARTICULARS 4.1 Therapeutic Indications Trademark Proprietor: Pfizer Products Inc. USA Licensed User: Pfizer Limited, India BECOSULES Capsules Page 1 of 7 LPDBCC092017 PfLEET Number: 2017-0033507 Becosules capsules are indicated in the treatment of patients with deficiencies of, or increased requirement for, vitamin B-complex, and vitamin C. Such patients and conditions include: Decreased intake because of restricted or unbalanced diet as in anorexia, diabetes mellitus, obesity and alcoholism. Reduced availability during treatment with antimicrobials which alter normal intestinal flora, in prolonged diarrhea and in chronic gastro-intestinal disorders. Increased requirements due to increased metabolic rate as in fever and tissue wasting, e.g. febrile illness, acute or chronic infections, surgery, burns and fractures. Stomatitis, glossitis, cheilosis, paraesthesias, neuralgia and dermatitis. Micronutrient deficiencies during pregnancy or lactation. -
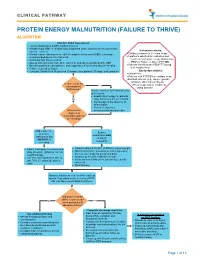
Protein Energy Malnutrition (Failure to Thrive) Algorithm
CLINICAL PATHWAY PROTEIN ENERGY MALNUTRITION (FAILURE TO THRIVE) ALGORITHM Conduct Initial Assessment • History and physical (H&P), nutrition focused • Weight height, BMI, % of ideal body weight and exam: assess severity (symmetric edema = severe) Inclusion criteria: • • Consider basic labs based on H&P; A complete blood count (CBC) is strongly Children newborn to 21 years of age • recommended due to risk of anemia Inpatients admitted for evaluation and • Additional labs based on H&P treatment of Protein Energy Malnutrition • Assess micronutrients: iron, zinc, vitamin D, and others as indicated by H&P (PEM) or Failure to thrive (FTT) OR • • Baseline potassium, phosphorus, and magnesium if concerned about re-feeding Patients identified with PEM/FTT during • Calorie count up to 3 days their hospital stay. • Consults: Social Work, Registered Dietician, Occupational Therapy, and Lactation Exclusion criteria: • Outpatients • Patients with FTT/PEM secondary to an identified concern (e.g., cancer, genetic condition, other chronic illness). Is there a risk for •Pts w/ suspected or confirmed micronutrient Yes eating disorder deficiencies? Initiate treatment for micronutrients deficiencies: • Empiric zinc therapy for patients No older than 6 months for 1 month • Iron therapy in the absence of inflammation • Vitamin D and other What are the micronutrients based on labs degrees of malnutrition and risk of refeeding? Mild, moderate, Severe or severe malnutrition AND malnutrition but at risk of NO RISK of refeeding refeeding • • Initiate feeding per recommended Initiate feeding at 30-50% of RDA for current weight • daily allowance (RDA) for current Monitor potassium, phosphorus, and magnesium weight and age once to twice a day for a total of 4 days • • Use PO route if patient is able to Advance by 10-20% if labs are normal • take 70% of estimated calories If labs abnormal hold off on advancing feed until orally corrected • Start thiamine Advance calories to meet level for catch up growth.