Neonatal Diabetes Mellitus and Congenital
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genomic Imprinting at the Porcine PLAGL1 Locus and the Orthologous Locus in the Human
G C A T T A C G G C A T genes Article Genomic Imprinting at the Porcine PLAGL1 Locus and the Orthologous Locus in the Human Jinsoo Ahn 1 , In-Sul Hwang 2 , Mi-Ryung Park 2, Seongsoo Hwang 2 and Kichoon Lee 1,* 1 Functional Genomics Laboratory, Department of Animal Sciences, The Ohio State University, Columbus, OH 43210, USA; [email protected] 2 Animal Biotechnology Division, National Institute of Animal Science, Rural Development Administration, Wanju, Jeonbuk 55365, Korea; [email protected] (I.-S.H.); [email protected] (M.-R.P.); [email protected] (S.H.) * Correspondence: [email protected]; Tel.: +1-614-688-7963 Abstract: Implementation of genomic imprinting in mammals often results in cis-acting silencing of a gene cluster and monoallelic expression, which are important for mammalian growth and function. Compared with widely documented imprinting status in humans and mice, current understanding of genomic imprinting in pigs is relatively limited. The objectives of this study were to identify DNA methylation status and allelic expression of alternative spliced isoforms at the porcine PLAGL1 locus and assess the conservation of the locus compared to the orthologous human locus. DNA methylome and transcriptome were constructed using porcine parthenogenetic or biparental control embryos. Using methylome, differentially methylated regions between those embryos were identified. Alternative splicing was identified by differential splicing analysis, and monoallelic expression was examined using single nucleotide polymorphism sites. Moreover, topological boundary regions were identified by analyzing CTCF binding sites and compared with the boundary of human orthologous locus. As a result, it was revealed that the monoallelic expression of the PLAGL1 Citation: Ahn, J.; Hwang, I.-S.; Park, M.-R.; Hwang, S.; Lee, K. -

Case Study: Testing for Genetic Disorders
Case study: Testing for genetic disorders Purpose: A genetic disorder is a disorder that is caused by an abnormality (or several abnormalities) in a person’s genome. Genetic disorders are often hereditary, which means they are passed down to a child from its parents. The most common genetic disorder is familial hypercholesterolaemia (a genetic predisposition to high cholesterol levels), which is thought to affect 1 in 250 people in the UK. Other disorders, such as cystic fibrosis, are rarer although they can be relatively common in particular ethnic groups; for instance, the incidence of cystic fibrosis in Scotland is almost double the global average. Collectively genetic disorders affect lots of people because there are more than 10,000 different types worldwide. Genetic testing is used to inform people whether they have a disorder, or if there is a chance they might pass a disorder on to their children. Photo credit: jxfzsy/ iStockphoto Photo credit: monkeybusiness/ iStockphoto Approaches to identifying genetic disorders In the UK, if a person is concerned they might have, or might develop, a genetic disorder based on their family history, they can: Go to their GP, who might refer them for genetic testing. Patients usually provide a DNA sample, which is then analysed for a specific abnormality. Usually a single gene will be tested, but whole-genome sequencing approaches are being developed. The 100,000 Genomes Project, for example, aims to study whole-genome sequences from patients with cancer and rare disease Use a commercial genetic testing service. In this case, customers collect samples of their own DNA and send them away for analysis and interpretation Take no action. -

DOI: 10.4274/Jcrpe.Galenos.2021.2020.0175
DOI: 10.4274/jcrpe.galenos.2021.2020.0175 Case report A novel SCNN1A variation in a patient with autosomal-recessive pseudohypoaldosteronism type 1 Mohammed Ayed Huneif1*, Ziyad Hamad AlHazmy2, Anas M. Shoomi 3, Mohammed A. AlGhofely 3, Dr Humariya Heena 5, Aziza M. Mushiba 4, Abdulhamid AlSaheel3 1Pediatric Endocrinologist at Najran university hospital, Najran Saudi Arabia. 2 Pediatric Endocrinologist at Al yamammah hospital, , Riyadh, Saudi Arabia. 3 Pediatric Endocrinologist at Pediatric endocrine department,. Obesity, Endocrine, and Metabolism Center, , King Fahad Medical City, Riyadh, Saudi Arabia. 4Clinical Geneticist, Pediatric Subspecialties Department, Children's Specialized Hospital, King Fahad Medical City, Riyadh, Saudi Arabia. 5 Research Center, King Fahad Medical City, Riyadh , Saudi Arabia What is already known on this topic ? Autosomal-recessive pseudohypoaldosteronism type 1 (PHA1) is a rare genetic disorder caused by different variations in the ENaC subunit genes. Most of these variations appear in SCNN1A mainly in exon eight, which encodes for the alpha subunit of the epithelial sodium channel ENaC. Variations are nonsense, single-base deletions or insertions, or splice site variations, leading to mRNA and proteins of abnormal length. In addition, a few new missense variations have been reported. What this study adds ? We report a novel mutation [ c.729_730delAG (p.Val245Glyfs*65) ] in the exon 4 of the SCNN1A gene In case of autosomal recessive pseudohypoaldosteronism type 1. Patient with PHA1 requires early recognition, proper treatment, and close follow-up. Parents are advised to seek genetic counseling and plan future pregnancies. proof Abstract Pseudohypoaldosteronism type 1 (PHA1) is an autosomal-recessive disorder characterized by defective regulation of body sodium levels. -

Zinc-Finger Protein 471 Suppresses Gastric Cancer Through
Oncogene (2018) 37:3601–3616 https://doi.org/10.1038/s41388-018-0220-5 ARTICLE Zinc-finger protein 471 suppresses gastric cancer through transcriptionally repressing downstream oncogenic PLS3 and TFAP2A 1 1 1 2 1 3 Lei Cao ● Shiyan Wang ● Yanquan Zhang ● Ka-Chun Wong ● Geicho Nakatsu ● Xiaohong Wang ● 1 3 1 Sunny Wong ● Jiafu Ji ● Jun Yu Received: 28 June 2017 / Revised: 23 December 2017 / Accepted: 23 February 2018 / Published online: 3 April 2018 © The Author(s) 2018. This article is published with open access Abstract Zinc-finger protein 471 (ZNF471) was preferentially methylated in gastric cancer using promoter methylation array. The role of ZNF471 in human cancer is unclear. Here we elucidated the functional significance, molecular mechanisms and clinical impact of ZNF471 in gastric cancer. ZNF471 mRNA was silenced in 15 out of 16 gastric cancer cell lines due to promoter hypermethylation. Significantly higher ZNF471 promoter methylation was also observed in primary gastric cancers compared to their adjacent normal tissues (P<0.001). ZNF471 promoter CpG-site hypermethylation correlated with poor 1234567890();,: survival of gastric cancer patients (n = 120, P = 0.001). Ectopic expression of ZNF471 in gastric cancer cell lines (AGS, BGC823, and MKN74) significantly suppressed cell proliferation, migration, and invasion, while it induced apoptosis in vitro and inhibited xenograft tumorigenesis in nude mice. Transcription factor AP-2 Alpha (TFAP2A) and plastin3 (PLS3) were two crucial downstream targets of ZNF471 demonstrated by bioinformatics modeling and ChIP-PCR assays. ZNF471 directly bound to the promoter of TFAP2A and PLS3 and transcriptionally inhibited their expression. TFAP2A and PLS3 showed oncogenic functions in gastric cancer cell lines. -

PLAGL1 (ZAC1/LOT1) Expression in Clear Cell Renal Cell Carcinoma: Correlations with Disease Progression and Unfavorable Prognosis
ANTICANCER RESEARCH 36: 617-624 (2016) PLAGL1 (ZAC1/LOT1) Expression in Clear Cell Renal Cell Carcinoma: Correlations with Disease Progression and Unfavorable Prognosis JANUSZ GODLEWSKI1, BARTLOMIEJ E. KRAZINSKI1, ANNA E. KOWALCZYK1, JOLANTA KIEWISZ1, JACEK KIEZUN1, PRZEMYSLAW KWIATKOWSKI1, AGNIESZKA SLIWINSKA-JEWSIEWICKA1, ZBIGNIEW MASLOWSKI2 and ZBIGNIEW KMIEC1,3 1Department of Human Histology and Embryology, Faculty of Medical Sciences, University of Warmia and Mazury in Olsztyn, Olsztyn, Poland; 2Department of Oncological Surgery, Warmia and Mazury Oncological Center, Olsztyn, Poland; 3Department of Histology, Medical University of Gdansk, Gdansk, Poland Abstract. Background: Pleiomorphic adenoma gene-like 1 Clear cell renal cell carcinoma (ccRCC), the prevailing form (PLAGL1) protein was originally shown to induce cell-cycle of RCC, is characterized by the most aggressive behavior and arrest and promote apoptosis in several types of human poor prognosis among all types of kidney cancer (1-3). tumors and therefore it was considered a candidate tumor Although ccRCC tumors can be removed surgically, suppressor. The involvement of PLAGL1 gene in the etiology haematogeneous metastases frequently occur in the early and pathogenesis of clear cell renal cell carcinoma (ccRCC) stage of the disease and 35-65% of patients develop has not been evaluated. The purpose of the present study metastatic disease after nephrectomy (3). Over the past was to determine the expression level of PLAGL1 in ccRCC decade, the background of ccRCC pathogenesis has been and to investigate its potential utility as a prognostic factor. extensively screened for molecular biomarkers and relevant Materials and Methods: We applied quantitative real-time gene signatures, however, only few have significant polymerase chain reaction (QPCR), western blotting and prognostic value which can be used in clinical practice (3-5). -

Inheriting Genetic Conditions
Help Me Understand Genetics Inheriting Genetic Conditions Reprinted from MedlinePlus Genetics U.S. National Library of Medicine National Institutes of Health Department of Health & Human Services Table of Contents 1 What does it mean if a disorder seems to run in my family? 1 2 Why is it important to know my family health history? 4 3 What are the different ways a genetic condition can be inherited? 6 4 If a genetic disorder runs in my family, what are the chances that my children will have the condition? 15 5 What are reduced penetrance and variable expressivity? 18 6 What do geneticists mean by anticipation? 19 7 What are genomic imprinting and uniparental disomy? 20 8 Are chromosomal disorders inherited? 22 9 Why are some genetic conditions more common in particular ethnic groups? 23 10 What is heritability? 24 Reprinted from MedlinePlus Genetics (https://medlineplus.gov/genetics/) i Inheriting Genetic Conditions 1 What does it mean if a disorder seems to run in my family? A particular disorder might be described as “running in a family” if more than one person in the family has the condition. Some disorders that affect multiple family members are caused by gene variants (also known as mutations), which can be inherited (passed down from parent to child). Other conditions that appear to run in families are not causedby variants in single genes. Instead, environmental factors such as dietary habits, pollutants, or a combination of genetic and environmental factors are responsible for these disorders. It is not always easy to determine whether a condition in a family is inherited. -

Restoration of Fertility by Gonadotropin Replacement in a Man With
J Rohayem and others Fertility in hypogonadotropic 170:4 K11–K17 Case Report CAH with TARTs Restoration of fertility by gonadotropin replacement in a man with hypogonadotropic azoospermia and testicular adrenal rest tumors due to untreated simple virilizing congenital adrenal hyperplasia Julia Rohayem1, Frank Tu¨ ttelmann2, Con Mallidis3, Eberhard Nieschlag1,4, Sabine Kliesch1 and Michael Zitzmann1 Correspondence should be addressed 1Center of Reproductive Medicine and Andrology, Clinical Andrology, University of Muenster, Albert-Schweitzer- to J Rohayem Campus 1, Building D11, D-48149 Muenster, Germany, 2Institute of Human Genetics and 3Institute of Reproductive Email and Regenerative Biology, Center of Reproductive Medicine and Andrology, University of Muenster, Muenster, Julia.Rohayem@ Germany and 4Center of Excellence in Genomic Medicine Research, King Abdulaziz University, Jeddah, Saudi Arabia ukmuenster.de Abstract Context: Classical congenital adrenal hyperplasia (CAH), a genetic disorder characterized by 21-hydroxylase deficiency, impairs male fertility, if insufficiently treated. Patient: A 30-year-old male was referred to our clinic for endocrine and fertility assessment after undergoing unilateral orchiectomy for a suspected testicular tumor. Histopathological evaluation of the removed testis revealed atrophy and testicular adrenal rest tumors (TARTs) and raised the suspicion of underlying CAH. The remaining testis was also atrophic (5 ml) with minor TARTs. Serum 17-hydroxyprogesterone levels were elevated, cortisol levels were at the lower limit of normal range, and gonadotropins at prepubertal levels, but serum testosterone levels were within the normal adult range. Semen analysis revealed azoospermia. CAH was confirmed by a homozygous mutation g.655A/COG (IVS2-13A/COG) in European Journal of Endocrinology CYP21A2. Hydrocortisone (24 mg/m2) administered to suppress ACTH and adrenal androgen overproduction unmasked deficient testicular testosterone production. -

A New Age in the Genetics of Deafness
, September/October 1999. Vol. 1 . No. 6 invited review/cme article A new age in the genetics of deafness Heidi L. Rehtu, MMSC' ot~dCytltllia C. Morton, PAD' Over the last several years, an understanding of the genetics SYNDROMIC AND NONSYNDROMIC DEAFNESS of hearing and deafness has grown exponentially. This progress , The prevalence of severe to profound bilateral congenital has been fueled by fast-paced developments in gene mapping hearing loss is estimated at 1 in 1000 births? When milder I and discovery, leading to the recent cloning and characteriza- forms of permanent hearing impairment at birth are included, tion of many genes critical in the biology of hearing. Among this estimate increases four-fold. Congenital deafness refers to the most significant advances, especially from a clinical per- deafness present at birth, though the cause of such hearing spective, is the discovery that up to 50% of nonsyndromic re- impairment may be genetic (hereditary) or environmental (ac- cessive deafness is caused by mutations in the GIB2 gene, which quired). Various studies estimate that approximately one-half encodes the connexin 26 protein (Cx26).I.' The clinical use of of the cases of congenital deafness are due to genetic factor^,^ screening for mutations in Cx26 and other genes involved in and these factors can be further subdivided by mode of inher- hearing impairment is still a new concept and not widely avail- itance. Approximately 77% of cases of hereditary deafness are able. However, the eventual availability of an array of genetic autosomal recessive, 22% are autosomal dominant, and 1% are tests is likely to have a major impact on the medical decisions X-linked.-l In addition, a small fraction of hereditary deafness made by hearing-impaired patients, their families, and their (< 1%) represents those families with mitochondria1 inheri- physicians. -

Waardenburg Syndrome: Case Series
International Journal of Otorhinolaryngology and Head and Neck Surgery Sachdeva S et al. Int J Otorhinolaryngol Head Neck Surg. 2021 Apr;7(4):668-671 http://www.ijorl.com pISSN 2454-5929 | eISSN 2454-5937 DOI: https://dx.doi.org/10.18203/issn.2454-5929.ijohns20211191 Case Series Waardenburg syndrome: case series Sheenu Sachdeva*, Varunkumar Jayakumar, Shubhlaxmi Atmaram Jaiswal Department of Otorhinolaryngology, Dr. V. M. G. M. C Solapur, Maharashtra, India Received: 13 January 2021 Revised: 16 February 2021 Accepted: 06 March 2021 *Correspondence: Dr. Sheenu Sachdeva, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Waardenburg syndrome is a rare genetic disorder of neural crest cell development with incidence of 1:42000 to 1:50,000. The syndrome is not completely expressed and hence adds to its hetergenecity with symptoms varying from one type of syndrome to another and from one patient to another. Unilateral heterochromia that manifests in some people is associated with Waardenburg syndrome and Parry-Romberg syndrome. This is a case series of four cases with features of Waardenburg syndrome with variable presentations and familial inheritance. Keywords: Autosomal dominant deafness, Heterochromia, Pigmentation anomalies, Waardenburg syndrome INTRODUCTION normal. Her mother also had similar hypertelorism with normal hearing (Figure 2). Also there is history suggestive Waardenburg syndrome (WS) is a rare genetic disorder of of premature graying of hair in her grandmother since the neural crest development characterized by varying degrees age of 15 years. -

Hearing Loss in Waardenburg Syndrome: a Systematic Review
Clin Genet 2016: 89: 416–425 © 2015 John Wiley & Sons A/S. Printed in Singapore. All rights reserved Published by John Wiley & Sons Ltd CLINICAL GENETICS doi: 10.1111/cge.12631 Review Hearing loss in Waardenburg syndrome: a systematic review Song J., Feng Y., Acke F.R., Coucke P., Vleminckx K., Dhooge I.J. Hearing J. Songa,Y.Fenga, F.R. Ackeb, loss in Waardenburg syndrome: a systematic review. P. Couckec,K.Vleminckxc,d Clin Genet 2016: 89: 416–425. © John Wiley & Sons A/S. Published by and I.J. Dhoogeb John Wiley & Sons Ltd, 2015 aDepartment of Otolaryngology, Xiangya Waardenburg syndrome (WS) is a rare genetic disorder characterized by Hospital, Central South University, Changsha, People’s Republic of China, hearing loss (HL) and pigment disturbances of hair, skin and iris. b Classifications exist based on phenotype and genotype. The auditory Department of Otorhinolaryngology, cDepartment of Medical Genetics, Ghent phenotype is inconsistently reported among the different Waardenburg types University/Ghent University Hospital, and causal genes, urging the need for an up-to-date literature overview on Ghent, Belgium, and dDepartment for this particular topic. We performed a systematic review in search for articles Biomedical Molecular Biology, Ghent describing auditory features in WS patients along with the associated University, Ghent, Belgium genotype. Prevalences of HL were calculated and correlated with the different types and genes of WS. Seventy-three articles were included, describing 417 individual patients. HL was found in 71.0% and was Key words: genotype – hearing loss – predominantly bilateral and sensorineural. Prevalence of HL among the inner ear malformation – phenotype – different clinical types significantly differed (WS1: 52.3%, WS2: 91.6%, Waardenburg syndrome WS3: 57.1%, WS4: 83.5%). -
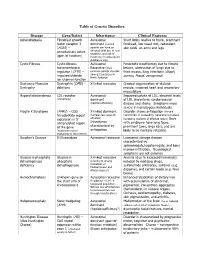
Table of Genetic Disorders Disease Gene/Defect Inheritance Clinical
Table of Genetic Disorders Disease Gene/Defect Inheritance Clinical Features Achondroplasia Fibroblast growth Autosomal Short limbs relative to trunk, prominent factor receptor 3 dominant (normal forehead, low nasal root, redundant (FGR3) – parents can have an skin folds on arms and legs constitutively active affected child due to new mutation, and risk of (gain of function) recurrence in subsequent children is low) Cystic Fibrosis Cystic fibrosis Autosomal Pancreatic insufficiency due to fibrotic transmembrane Recessive (most lesions, obstruction of lungs due to regulator (CFTR) – common genetic disorder thick mucus, lung infections (Staph, impaired chloride among Caucasians in aureus, Pseud. aeruginosa) North America) ion channel function Duchenne Muscular Dystrophin (DMD) - X-linked recessive Gradual degeneration of skeletal Dystrophy deletions muscle, impaired heart and respiratory musculature Hypercholesterolemia LDL receptor Autosomal Impaired uptake of LDL, elevated levels (commonly) dominant of LDL cholesterol, cardiovascular (haploinsufficiency) disease and stroke. Symptoms more severe in homozygous individuals Fragile X Syndrome (FMR1) – CGG X-linked dominant Disorder shows anticipation (female trinucleotide repeat (females less severely transmitters in succeeding generations produce expansion in 5’ affected) increasing numbers of affected males) Boys untranslated region Inheritance with syndrome have long faces, of the gene characterized by prominent jaws, large ears, and are (expansion occurs anticipation likely to be mentally retarded. exclusively in the mother) Gaucher’s Disease Β-Glucosidase Autosomal recessive Lysosomal storage disease characterized by splenomegaly,hepatomegaly, and bone marrow infiltration. Neurological symptoms are not common Glucose 6-phosphate Glucose 6- X-linked recessive Anemia (due to increased hemolysis) dehydrogenase phosphate (prominent among induced by oxidizing drugs, deficiency dehydrogenase individuals of sulfonamide antibiotics, sulfones (e.g. -

Altered Expression of the PLAGL1 (ZAC1/LOT1) Gene in Colorectal Cancer: Correlations to the Clinicopathological Parameters
INTERNATIONAL JOURNAL OF ONCOLOGY 47: 951-962, 2015 Altered expression of the PLAGL1 (ZAC1/LOT1) gene in colorectal cancer: Correlations to the clinicopathological parameters ANNA E. KowalcZYK1, Bartlomiej E. KRAZINSKI1, JANUSZ GODLEWSKI1, Jolanta KIEWISZ1, PRZEMYSlaw KwiatkowSKI1, AGNIESZKA SLIWINSKA-JEWSIEWICKA1, JACEK KIEZUN1, PIOTR M. WIERZBICKI2, GABRIEL BODEK3, MARIAN SULIK4 and ZBIGNIEW KMIEC1,2 1Department of Human Histology and Embryology, Faculty of Medical Sciences, University of Warmia and Mazury, 10-082 Olsztyn; 2Department of Histology, Medical University of Gdansk, 80-210 Gdansk; 3In Vitro and Cell Biotechnology Laboratory, Institute of Animal Reproduction and Food Research, Polish Academy of Sciences, 10-243 Olsztyn; 4Department of Pathomorphology, Faculty of Medical Sciences, University of Warmia and Mazury, 10-561 Olsztyn, Poland Received April 23, 2015; Accepted June 2, 2015 DOI: 10.3892/ijo.2015.3067 Abstract. Pleomorphic adenoma gene-like 1 gene (PLAGL1) late significantly with patient overall survival; however, the encodes a zinc-finger nuclear transcription factor which hazard ratio for patients whose tumor tissues showed reduced promotes apoptosis and cell cycle arrest. Loss or downregu- PLAGL1 immunohistochemical staining was twice higher lation of its expression has been observed in various human than in patients with increased PLAGL1 immunoreactivity. In neoplasms. This study compared PLAGL1 expression in conclusion, these results suggest that dysregulation of PLAGL1 colorectal cancer (CRC) tissue and colon mucosa of healthy expression may be involved to some extent in the progression subjects at the mRNA and protein levels, and estimated its of CRC, but the so far collected patient survival data do not prognostic value. The PLAGL1 mRNA levels were also deter- confirm applicability of the PLAGL1 expression level as a mined in CRC cell lines.