Integumentary Studies the Polycarpicae. IV. Liriodendron Tulipifera L. Rather Generally Accepted Primitive Angiospermous by Embr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Scientific Name Common Name NATURAL ASSOCIATIONS of TREES and SHRUBS for the PIEDMONT a List
www.rainscapes.org NATURAL ASSOCIATIONS OF TREES AND SHRUBS FOR THE PIEDMONT A list of plants which are naturally found growing with each other and which adapted to the similar growing conditions to each other Scientific Name Common Name Acer buergeranum Trident maple Acer saccarum Sugar maple Acer rubrum Red Maple Betula nigra River birch Trees Cornus florida Flowering dogwood Fagus grandifolia American beech Maple Woods Liriodendron tulipifera Tulip-tree, yellow poplar Liquidamber styraciflua Sweetgum Magnolia grandiflora Southern magnolia Amelanchier arborea Juneberry, Shadbush, Servicetree Hamamelis virginiana Autumn Witchhazel Shrubs Ilex opaca American holly Ilex vomitoria*** Yaupon Holly Viburnum acerifolium Maple leaf viburnum Aesulus parvilflora Bottlebrush buckeye Aesulus pavia Red buckeye Carya ovata Shadbark hickory Cornus florida Flowering dogwood Halesia carolina Crolina silverbell Ilex cassine Cassina, Dahoon Ilex opaca American Holly Liriodendron tulipifera Tulip-tree, yellow poplar Trees Ostrya virginiana Ironwood Prunus serotina Wild black cherry Quercus alba While oak Quercus coccinea Scarlet oak Oak Woods Quercus falcata Spanish red oak Quercus palustris Pin oak Quercus rubra Red oak Quercus velutina Black oak Sassafras albidum Sassafras Azalea nudiflorum Pinxterbloom azalea Azalea canescens Piedmont azalea Ilex verticillata Winterberry Kalmia latifolia Mountain laurel Shrubs Rhododenron calendulaceum Flame azalea Rhus copallina Staghorn sumac Rhus typhina Shining sumac Vaccinium pensylvanicum Low-bush blueberry Magnolia -

Yellow-Poplar: Characteristics and Management
Forest Service Agriculture Handbook Number 583 Yellow-Poplar: Characteristics and Management by Donald E. Beck, Principal Silviculturist and Lino Della-Bianca, Silviculturist Southeastern Forest Experiment Station Asheville, North Carolina Agriculture Handbook No. 583 U.S. Department of Agriculture Forest Service September 198i For sale by the Superintendent of Documents. U.S. Government Prlntlng Ofece Washlnxton. D.C. 20402 Library of Congress Catalog Card Number: 81-600045 This publication reports research involving pesticides. It does not contain recommendations for their use, nor does it imply that the uses discussed here have been registered. All uses of pesticides must be registered by appropriate State and/or Federal agencies before they can be recommended. CAUTION: Pesticides can be injurious to humans, domestic animals, desirable plants, and fish or other wildlife-if they are not handled or applied properly. Use all pesticides selectively and care- fully. Follow recommended practices for the disposal of surplus pesticides and pesticide containers. The use of trade, firm, or corporation names in this publication is for the information and convenience of the reader. Such use does not constitute endorsement or approval by the U.S. Department of Agriculture of any product or service to the exclusion of others which may be suitable. 2 Beck, Donald E., and Lino Della-Bianca. 1981. Yellow-poplar: Characteristics and management. U.S. Dep. Agric., Agric. Handb. 583, 92 p. This reference tool and field guide for foresters and other land managers includes a synthesis of information on the characteristics of yellow-poplar with guidelines for managing the species. It is based on research conducted by many individuals in State and Federal forestry organizations and in universities throughout the Eastern United States. -

Tuliptree: Liriodendron Tulipifera Family: Magnoliaceae
Pests: Athough there is not a pest that is damaging Tuliptree to the Tuliptree, the aphid can be a problem. As it feeds on new growth it leaves a sticky Liriodendron Tulipifera substance on the leaves called honeydew. Family: Magnoliaceae REFERENCES This sticky material is food heaven for mold and leads to black fungus on the leaves, not pretty!(2) 1.Arbor Day Foundation. (). Tuliptree . In www.arborday.org. Retrieved Sept. 18, 2011, from http://www.arborday.org/programs/ nationaltree/tuliptree.cfm. 2. Department of Natural Resources. (). Tuliptree (Liriodendron tulipifera). In www.dnr.state.oh.us. Economic importance: Retrieved Sept. 18, 2011, from http:// Due to the light and soft nature of the Tuliptree www.dnr.state.oh.us/Home/trees/tuliptree/ wood, it is ideal for wood crafting and is used tabid/5428/Default.aspx. 3. Fara, Heather. (). Tulip-tree, tuliptree magnolia, commercially for furniture, toys, and other tulip poplar, yellow poplar . In flmnh.ufl.edu. Re- wood products. In the lumber industry it is trieved Sept. 18, 2011, from http:// know as yellow poplar. It is also sold as shade www.flmnh.ufl.edu/flowerpower/poplar.html. and decorative trees where lots of space is 4.Grimm, W. C. (2002) Trees Mechanicsburg, PA. available for the fast growing, large tree. (4) Stackpole Books. 5. OPLIN. (1997-2001). Tuliptree (Yellow poplar). In www.oplin.org. Retrieved Sept. 18, 2011, from http://www.oplin.org/tree/fact%20pages/ tulip_tree/tulip_tree.html. 6. Wcisel, Karen. (2004-2009). Tuliptree fact Sheet. In treetopics.com. Retrieved Sept. 18, 2011, from http://www.treetopics.com/ liriodendron_tulipifera/index.htm. -

Tulip Tree Liriodendron Tulipifera
Tulip Tree Liriodendron tulipifera Liriodendron tulipifera is one of the tallest trees in the eastern United States. Its native range extends from Connecticut down the eastern seaboard to northern Florida, west to Louisiana, Tennessee, Indiana, and southern Illinois. In fact, it’s the state tree of Indiana, where enormous specimens can be found with trunks up to five feet in diameter and heights in excess of 100 feet. The tree is excurrent in growth habit, having a strong central leader. Mature tulip trees often lack branches for 50 or more feet from the ground, as if the main trunk were racing toward the sunlight. The leaf’s outline strongly resembles that of a tulip, hence not only the Latin name, but also the common names of tulip tree or tulip poplar. This species is also sometimes called yellow poplar, although it is not at all related to the trees of the Populus genus such as poplars, cottonwoods, or aspens. The tulip tree’s leaves turn a brilliant yellow in the fall, which makes the species an excellent landscape centerpiece. Early yellowing of the leaves in mid-summer, however, can be a sign of stress caused by drought or poor soil conditions. Liriodendron tulipifera is a member of the magnolia family, known for showy flowers. The tulip tree is no exception, boasting erect, cup-shaped flowers that are green-ish yellow with orange bases that bloom in May. The tree also has distinctive, flattened “duckbill” buds. This is a good winter identification tool, if there are branches low enough for the buds to be seen. -

Upper Canopy Collection and Identification of Grapevines (Vitis) from Selected Forests in the Southeastern United States Sydney E
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Papers in Plant Pathology Plant Pathology Department 2010 Upper Canopy Collection and Identification of Grapevines (Vitis) from Selected Forests in the Southeastern United States Sydney E. Everhart University of Nebraska-Lincoln, [email protected] Follow this and additional works at: http://digitalcommons.unl.edu/plantpathpapers Part of the Other Plant Sciences Commons, Plant Biology Commons, and the Plant Pathology Commons Everhart, Sydney E., "Upper Canopy Collection and Identification of Grapevines (Vitis) from Selected Forests in the Southeastern United States" (2010). Papers in Plant Pathology. 364. http://digitalcommons.unl.edu/plantpathpapers/364 This Article is brought to you for free and open access by the Plant Pathology Department at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Papers in Plant Pathology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. CASTANEA 75(1): 141–149. MARCH 2010 Upper Canopy Collection and Identification of Grapevines (Vitis) from Selected Forests in the Southeastern United States Sydney E. Everhart* Department of Biology and Earth Science, University of Central Missouri, Warrensburg, Missouri 64093 ABSTRACT Woody grapevines (Vitis spp.) are common in the deciduous forests of the southeastern United States. Their growth habit makes leaf collection challenging and polymorphic leaves make identification of species difficult. Mature grapevines can grow up to 48 cm in diameter at breast height and reach the upper canopy of trees more than 35 m in height. Leaf morphology is the most readily available character used for species identification. However, most mature grapevines do not produce leaves below the upper canopy and if they do, these leaves are morphologically indistinguishable from other species. -

NLI Recommended Plant List for the Mountains
NLI Recommended Plant List for the Mountains Notable Features Requirement Exposure Native Hardiness USDA Max. Mature Height Max. Mature Width Very Wet Very Dry Drained Moist &Well Occasionally Dry Botanical Name Common Name Recommended Cultivars Zones Tree Deciduous Large (Height: 40'+) Acer rubrum red maple 'October Glory'/ 'Red Sunset' fall color Shade/sun x 2-9 75' 45' x x x fast growing, mulit-stemmed, papery peeling Betula nigra river birch 'Heritage® 'Cully'/ 'Dura Heat'/ 'Summer Cascade' bark, play props Shade/part sun x 4-8 70' 60' x x x Celtis occidentalis hackberry tough, drought tolerant, graceful form Full sun x 2-9 60' 60' x x x Fagus grandifolia american beech smooth textured bark, play props Shade/part sun x 3-8 75' 60' x x Fraxinus americana white ash fall color Full sun/part shade x 3-9 80' 60' x x x Ginkgo biloba ginkgo; maidenhair tree 'Autumn Gold'/ 'The President' yellow fall color Full sun 3-9 70' 40' x x good dappled shade, fall color, quick growing, Gleditsia triacanthos var. inermis thornless honey locust Shademaster®/ Skyline® salt tolerant, tolerant of acid, alkaline, wind. Full sun/part shade x 3-8 75' 50' x x Liriodendron tulipifera tulip poplar fall color, quick growth rate, play props, Full sun x 4-9 90' 50' x Platanus x acerifolia sycamore, planetree 'Bloodgood' play props, peeling bark Full sun x 4-9 90' 70' x x x Quercus palustris pin oak play props, good fall color, wet tolerant Full sun x 4-8 80' 50' x x x Tilia cordata Little leaf Linden, Basswood 'Greenspire' Full sun/part shade 3-7 60' 40' x x Ulmus -
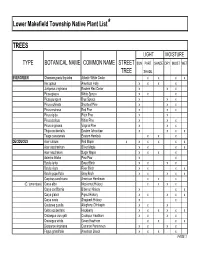
Native Plant List Trees.XLS
Lower Makefield Township Native Plant List* TREES LIGHT MOISTURE TYPE BOTANICAL NAME COMMON NAME STREET SUN PART SHADE DRY MOIST WET TREE SHADE EVERGREEN Chamaecyparis thyoides Atlantic White Cedar x x x x IIex opaca American Holly x x x x Juniperus virginiana Eastern Red Cedar x x x Picea glauca White Spruce x x x Picea pungens Blue Spruce x x x Pinus echinata Shortleaf Pine x x x Pinus resinosa Red Pine x x x Pinus rigida Pitch Pine x x Pinus strobus White Pine x x x Pinus virginiana Virginia Pine x x x Thuja occidentalis Eastern Arborvitae x x x x Tsuga canadensis Eastern Hemlock xx x DECIDUOUS Acer rubrum Red Maple x x x x x x Acer saccharinum Silver Maple x x x x Acer saccharum Sugar Maple x x x x Asimina triloba Paw-Paw x x Betula lenta Sweet Birch x x x x Betula nigra River Birch x x x x Betula populifolia Gray Birch x x x x x Carpinus caroliniana American Hornbeam x x x (C. tomentosa) Carya alba Mockernut Hickory x x x x Carya cordiformis Bitternut Hickory x x x Carya glabra Pignut Hickory x x x x x Carya ovata Shagbark Hickory x x Castanea pumila Allegheny Chinkapin xx x Celtis occidentalis Hackberry x x x x x x Crataegus crus-galli Cockspur Hawthorn x x x x Crataegus viridis Green Hawthorn x x x x Diospyros virginiana Common Persimmon x x x x Fagus grandifolia American Beech x x x x PAGE 1 Exhibit 1 TREES (cont'd) LIGHT MOISTURE TYPE BOTANICAL NAME COMMON NAME STREET SUN PART SHADE DRY MOIST WET TREE SHADE DECIDUOUS (cont'd) Fraxinus americana White Ash x x x x Fraxinus pennsylvanica Green Ash x x x x x Gleditsia triacanthos v. -

Liriodendron Tulipifera Tuliptree1 Edward F
Fact Sheet ST-363 November 1993 Liriodendron tulipifera Tuliptree1 Edward F. Gilman and Dennis G. Watson2 INTRODUCTION Tuliptree grows 80 to 100 feet tall and maintains a fairly narrow oval crown, even as it grows older (Fig. 1). Trunks become massive in old age, becoming deeply furrowed with thick bark. The tree maintains a straight trunk and generally does not form double or multiple leaders. Older trees have several large- diameter major limbs in the top half of the crown. Tuliptree has a moderate to rapid (on good sites) growth rate at first but slows down with age. The soft wood reportedly is subject to storm damage but the trees held up remarkably well in the south during hurricane ‘Hugo’. It is probably stronger than given credit for. The largest trees in the east are in the Joyce Kilmer Forest in NC, some reaching more than 150 feet with seven-foot diameter trunks. The fall color is gold to yellow being more pronounced in the northern part of its range. The scented, tulip-like, greenish-yellow flowers appear in mid-spring but are not as ornamental as those of other flowering trees because they are far from view. GENERAL INFORMATION Scientific name: Liriodendron tulipifera Pronunciation: leer-ee-oh-DEN-drawn too-lih-PIFF-er-uh Common name(s): Tuliptree, Tulip-Poplar, Figure 1. Middle-aged Tuliptree. Yellow-Poplar Family: Magnoliaceae Availability: generally available in many areas within USDA hardiness zones: 5 through 9A (Fig. 2) its hardiness range Origin: native to North America Uses: shade tree; no proven urban tolerance 1. -

Landscaping Near Black Walnut Trees
Selecting juglone-tolerant plants Landscaping Near Black Walnut Trees Black walnut trees (Juglans nigra) can be very attractive in the home landscape when grown as shade trees, reaching a potential height of 100 feet. The walnuts they produce are a food source for squirrels, other wildlife and people as well. However, whether a black walnut tree already exists on your property or you are considering planting one, be aware that black walnuts produce juglone. This is a natural but toxic chemical they produce to reduce competition for resources from other plants. This natural self-defense mechanism can be harmful to nearby plants causing “walnut wilt.” Having a walnut tree in your landscape, however, certainly does not mean the landscape will be barren. Not all plants are sensitive to juglone. Many trees, vines, shrubs, ground covers, annuals and perennials will grow and even thrive in close proximity to a walnut tree. Production and Effect of Juglone Toxicity Juglone, which occurs in all parts of the black walnut tree, can affect other plants by several means: Stems Through root contact Leaves Through leakage or decay in the soil Through falling and decaying leaves When rain leaches and drips juglone from leaves Nuts and hulls and branches onto plants below. Juglone is most concentrated in the buds, nut hulls and All parts of the black walnut tree produce roots and, to a lesser degree, in leaves and stems. Plants toxic juglone to varying degrees. located beneath the canopy of walnut trees are most at risk. In general, the toxic zone around a mature walnut tree is within 50 to 60 feet of the trunk, but can extend to 80 feet. -

Liriodendron Tulipifera (Tulip Poplar) Magnolia Family (Magnoliaceae)
Liriodendron tulipifera (Tulip Poplar) Magnolia Family (Magnoliaceae) Introduction: Tulip poplar is one of the tallest of the native American hardwoods. Kentucky was home to some of the most magnificent of these stately trees. Kentucky, Tennessee and Indiana have named tulip poplar as the state tree. The tree has winter features including duck's bill-shaped buds and furrowed bark. It also offers striking flowers in May and June. Leaves emerge folded and yellow and become green with age. They turn a clear yellow in autumn. Culture: Tulip poplar thrives in deep, rich, well-drained but moist soil and full sun. It is pH adaptable but performs best in soil that is slightly acidic. This tree is sensitive to drought and may require summer irrigation to prevent early leaf abscission. It should be transplanted balled-and-burlapped in spring. Tulip poplar is susceptible to Verticillium wilt, and may be bothered by tulip tree leaf miner (sassafras weevil). Aphids may feed on the foliage and the insects’ sticky exudate (and the black sooty mold that grows on the exudate) drops on whatever is under the tree. Because some trees may be particularly weak- wooded, ice storms and wind may cause significant damage. Selected cultivars: ‘Ardis’ - A dwarf tree, one-third the size of the species. Botanical Characteristics: ‘Aureomarginatum’ - One of the few cultivars commercially Native habitat - Eastern U.S. in deciduous woods. available. Variegated, with a yellow leaf edge and green center; Growth habit - In the wild, this tree is slower-growing than the species. known for its straight trunk and high ‘Fastigiatum’ - Narrow form, with upright branches nearly canopy. -

FREE Street Tree Planting Program
Whitpain Township Shade Tree Commission FREE Street Tree Planting Program Street Trees provide many ecological and aesthetic services which benefit our Whitpain Township community. Shading our streets and creating a harmonious landscape are among the greatest benefits that increase the value of our properties and sense of community pride. For these and many more reasons the Shade Tree Commission has created the Whitpain Township Free Street Tree Program to promote the establishment of new street trees which will benefit our community for generation to come and increase property values. The Free Street Tree Program is a pilot program brought to you by the Whitpain Township shade Tree Commission and the Board of Supervisors. There are a limited number of trees available this year, so this application is not a guarantee of receiving a tree. Through this vital program Whitpain Township property owners will be able to obtain a free street tree, 2”-2.5”Cal., for their property, if they meet all the criteria in the application, planted by approved contractors partnering with the Shade Tree Commission. The FREE Street Tree Program is limited, one street tree will be provided to the first qualifying applicants each year. Qualifying Criteria for a Free Street Tree Proposed street tree location must be within 8 feet of the street or sidewalk adjacent to the street. The street tree location must be clear of all underground and overhead utilities. Homeowners are required call PA One Call to have utilities marked in advance of tree planting. This is a free service. Home-owner assumes all responsibility for the Street Tree including but not limited to regular thorough watering during the initial two year establishment period. -

EVALUATING DIEBACK of EASTERN WHITE PINE (Pinus Strobus L.) in THE
EVALUATING DIEBACK OF EASTERN WHITE PINE (Pinus strobus L.) IN THE SOUTHERN APPALACHIAN MOUNTAINS by ASHLEY NICOLE SCHULZ (Under the Direction of Kamal J.K. Gandhi) ABSTRACT During the last decade, Pinus strobus L. trees in the Appalachian Mountain region of the United States have been displaying symptoms of dieback, including branch flagging, resinosis, and crown thinning. Many of these economically and ecologically important trees also have a scale insect, Matsucoccus macrocicatrices Richards, and various fungal pathogens associated with canker formation. For this study, we evaluated the health of P. strobus in 40 sites across the southern Appalachian Mountains, modeled the relationships between P. strobus health and abiotic and biotic conditions, and assessed correlations among P. strobus saplings, M. macrocicatrices, and cankers. Overall, we found that M. macrocicatrices and a canker-forming fungus, Caliciopsis pinea Peck, were present in 85% and 87.5% of the 40 sites, respectively. Pinus strobus health rating was associated with DBH, tree density, and latitude, where larger diameter P. strobus trees in less dense stands were healthier than smaller diameter trees in denser stands, and trees in Virginia were less healthy than trees in Georgia. Positive correlations were present within the tripartite, P. strobus-M. macrocicatrices-canker complex, suggesting that sapling dieback is associated with M. macrocicatrices and cankers. Further exploration of the relationships among M. macrocicatrices, cankers, and site conditions are encouraged