EVALUATING DIEBACK of EASTERN WHITE PINE (Pinus Strobus L.) in THE
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Scientific Name Species Common Name Abies Lasiocarpa FIR Subalpine Acacia Macracantha ACACIA Long-Spine
Scientific Name Species Common Name Abies lasiocarpa FIR Subalpine Acacia macracantha ACACIA Long-spine Acacia roemeriana CATCLAW Roemer Acer grandidentatum MAPLE Canyon Acer nigrum MAPLE Black Acer platanoides MAPLE Norway Acer saccharinum MAPLE Silver Aesculus pavia BUCKEYE Red Aesculus sylvatica BUCKEYE Painted Ailanthus altissima AILANTHUS Tree-of-heaven Albizia julibrissin SILKTREE Mimosa Albizia lebbek LEBBEK Lebbek Alnus iridis ssp. sinuata ALDER Sitka Alnus maritima ALDER Seaside Alvaradoa amorphoides ALVARADOA Mexican Amelanchier laevis SERVICEBERRY Allegheny Amyris balsamifera TORCHWOOD Balsam Annona squamosa SUGAR-APPLE NA Araucaria cunninghamii ARAUCARIA Cunningham Arctostaphylos glauca MANZANITA Bigberry Asimina obovata PAWPAW Bigflower Bourreria radula STRONGBACK Rough Brasiliopuntia brasiliensis PRICKLY-PEAR Brazilian Bursera simaruba GUMBO-LIMBO NA Caesalpinia pulcherrima FLOWERFENCE NA Capparis flexuosa CAPERTREE Limber CRUCIFIXION- Castela emoryi THORN NA Casuarina equisetifolia CASUARINA Horsetail Ceanothus arboreus CEANOTHUS Feltleaf Ceanothus spinosus CEANOTHUS Greenbark Celtis lindheimeri HACKBERRY Lindheimer Celtis occidentalis HACKBERRY Common Cephalanthus occidentalis BUTTONBUSH Common Cercis canadensis REDBUD Eastern Cercocarpus traskiae CERCOCARPUS Catalina Chrysophyllum oliviforme SATINLEAF NA Citharexylum berlandieri FIDDLEWOOD Berlandier Citrus aurantifolia LIME NA Citrus sinensis ORANGE Orange Coccoloba uvifera SEAGRAPE NA Colubrina arborescens COLUBRINA Coffee Colubrina cubensis COLUBRINA Cuba Condalia globosa -

Scientific Name Common Name NATURAL ASSOCIATIONS of TREES and SHRUBS for the PIEDMONT a List
www.rainscapes.org NATURAL ASSOCIATIONS OF TREES AND SHRUBS FOR THE PIEDMONT A list of plants which are naturally found growing with each other and which adapted to the similar growing conditions to each other Scientific Name Common Name Acer buergeranum Trident maple Acer saccarum Sugar maple Acer rubrum Red Maple Betula nigra River birch Trees Cornus florida Flowering dogwood Fagus grandifolia American beech Maple Woods Liriodendron tulipifera Tulip-tree, yellow poplar Liquidamber styraciflua Sweetgum Magnolia grandiflora Southern magnolia Amelanchier arborea Juneberry, Shadbush, Servicetree Hamamelis virginiana Autumn Witchhazel Shrubs Ilex opaca American holly Ilex vomitoria*** Yaupon Holly Viburnum acerifolium Maple leaf viburnum Aesulus parvilflora Bottlebrush buckeye Aesulus pavia Red buckeye Carya ovata Shadbark hickory Cornus florida Flowering dogwood Halesia carolina Crolina silverbell Ilex cassine Cassina, Dahoon Ilex opaca American Holly Liriodendron tulipifera Tulip-tree, yellow poplar Trees Ostrya virginiana Ironwood Prunus serotina Wild black cherry Quercus alba While oak Quercus coccinea Scarlet oak Oak Woods Quercus falcata Spanish red oak Quercus palustris Pin oak Quercus rubra Red oak Quercus velutina Black oak Sassafras albidum Sassafras Azalea nudiflorum Pinxterbloom azalea Azalea canescens Piedmont azalea Ilex verticillata Winterberry Kalmia latifolia Mountain laurel Shrubs Rhododenron calendulaceum Flame azalea Rhus copallina Staghorn sumac Rhus typhina Shining sumac Vaccinium pensylvanicum Low-bush blueberry Magnolia -

Yellow-Poplar: Characteristics and Management
Forest Service Agriculture Handbook Number 583 Yellow-Poplar: Characteristics and Management by Donald E. Beck, Principal Silviculturist and Lino Della-Bianca, Silviculturist Southeastern Forest Experiment Station Asheville, North Carolina Agriculture Handbook No. 583 U.S. Department of Agriculture Forest Service September 198i For sale by the Superintendent of Documents. U.S. Government Prlntlng Ofece Washlnxton. D.C. 20402 Library of Congress Catalog Card Number: 81-600045 This publication reports research involving pesticides. It does not contain recommendations for their use, nor does it imply that the uses discussed here have been registered. All uses of pesticides must be registered by appropriate State and/or Federal agencies before they can be recommended. CAUTION: Pesticides can be injurious to humans, domestic animals, desirable plants, and fish or other wildlife-if they are not handled or applied properly. Use all pesticides selectively and care- fully. Follow recommended practices for the disposal of surplus pesticides and pesticide containers. The use of trade, firm, or corporation names in this publication is for the information and convenience of the reader. Such use does not constitute endorsement or approval by the U.S. Department of Agriculture of any product or service to the exclusion of others which may be suitable. 2 Beck, Donald E., and Lino Della-Bianca. 1981. Yellow-poplar: Characteristics and management. U.S. Dep. Agric., Agric. Handb. 583, 92 p. This reference tool and field guide for foresters and other land managers includes a synthesis of information on the characteristics of yellow-poplar with guidelines for managing the species. It is based on research conducted by many individuals in State and Federal forestry organizations and in universities throughout the Eastern United States. -

Home, Yard Garden Pest
UNIVERSITY OF ILLINOIS EXTENSION HOME, Yard Garden Pest College of Agricultural, Consumer and Environmental Sciences, University of Illinois at Urbana-Champaign &Illinois Natural History Survey, Champaign NEWSLETTER No. 14 • August 6, 2008 Cedar–Quince Rust Affects Hawthorn Each spring we discuss cedar–apple and related rusts. In PLANT DISEASES________ the landscape, we are especially concerned with control- Diplodia of Pine Prevalent ling cedar–quince rust on hawthorn (issue no. 3, 2008) Diplodia tip blight is prevalent in much of Illinois this because it causes stem cankers and fruit infection. The summer. Take a look around your town, and you likely cankers cause stem tips to die. The fruit infection is at- will see many pines with brown needles at the tips of tractive only to a plant pathologist. Repeated rain events branches. Entire needles are brown, not just tips of nee- this spring allowed repeated waves of spore movement dles as might occur with scorch, salt injury, or transplant and germination from the alternate host (junipers) to shock. Look more closely at the needles, and you may the susceptible hawthorns. Secondary spread is by myce- see fungal fruiting bodies. They are often most prevalent lia only, and that growth is very limited. In many cases, at the base of needles. If fruiting bodies are not visible, hawthorn fruits have been covered with the aecial fruit- place the needles in a plastic bag with some moist (not ing bodies of this fungus. You can see the aecial spores dripping wet) paper toweling; blow some air into the which have washed or blown onto the foliage. -

Tuliptree: Liriodendron Tulipifera Family: Magnoliaceae
Pests: Athough there is not a pest that is damaging Tuliptree to the Tuliptree, the aphid can be a problem. As it feeds on new growth it leaves a sticky Liriodendron Tulipifera substance on the leaves called honeydew. Family: Magnoliaceae REFERENCES This sticky material is food heaven for mold and leads to black fungus on the leaves, not pretty!(2) 1.Arbor Day Foundation. (). Tuliptree . In www.arborday.org. Retrieved Sept. 18, 2011, from http://www.arborday.org/programs/ nationaltree/tuliptree.cfm. 2. Department of Natural Resources. (). Tuliptree (Liriodendron tulipifera). In www.dnr.state.oh.us. Economic importance: Retrieved Sept. 18, 2011, from http:// Due to the light and soft nature of the Tuliptree www.dnr.state.oh.us/Home/trees/tuliptree/ wood, it is ideal for wood crafting and is used tabid/5428/Default.aspx. 3. Fara, Heather. (). Tulip-tree, tuliptree magnolia, commercially for furniture, toys, and other tulip poplar, yellow poplar . In flmnh.ufl.edu. Re- wood products. In the lumber industry it is trieved Sept. 18, 2011, from http:// know as yellow poplar. It is also sold as shade www.flmnh.ufl.edu/flowerpower/poplar.html. and decorative trees where lots of space is 4.Grimm, W. C. (2002) Trees Mechanicsburg, PA. available for the fast growing, large tree. (4) Stackpole Books. 5. OPLIN. (1997-2001). Tuliptree (Yellow poplar). In www.oplin.org. Retrieved Sept. 18, 2011, from http://www.oplin.org/tree/fact%20pages/ tulip_tree/tulip_tree.html. 6. Wcisel, Karen. (2004-2009). Tuliptree fact Sheet. In treetopics.com. Retrieved Sept. 18, 2011, from http://www.treetopics.com/ liriodendron_tulipifera/index.htm. -

Dry White Pine (Hemlock) Oak Forest
Dry white pine (hemlock) oak forest This community type occurs on fairly dry sites, often with 25% or more of the forest floor covered by rocks, boulders and/or exposed bedrock. The canopy may be somewhat open and tree growth somewhat suppressed. The tree stratum is dominated by a mixture of Pinus strobus (eastern white pine), or occasionally Tsuga canadensis (eastern hemlock), and a mixture of dry-site hardwoods, predominantly oaks. On most sites, the conifer and the hardwood component both range between 25% and 75% of the canopy. The oak species most often associated with this type are Quercus montana (chestnut oak), and Q. alba (white oak), although Q. velutina (black oak), Q. coccinea (scarlet oak), or Q. rubra (northern red oak) may also occur. Other associated trees include Nyssa sylvatica (black-gum), Betula lenta (sweet birch), Fraxinus americana (white ash), Prunus serotina (wild black cherry), and Castanea dentata (American chestnut) sprouts. There is often a heath-dominated shrub layer with Kalmia latifolia (mountain laurel) being especially important; Gaylussacia baccata (black huckleberry), Vaccinium spp. (blueberries), and Kalmia angustifolia (sheep laurel) are also common. Other shrubs, like Cornus florida (flowering dogwood), Hamamelis virginiana (witch-hazel), Viburnum acerifolium (maple-leaved viburnum) may also occur on less acidic sites. There is typically a sparse herbaceous layer with a northern affinity; Aralia nudicaulis (wild sarsaparilla), Pteridium aquilinum (bracken fern), Maianthemum canadense (Canada mayflower), Gaultheria procumbens (teaberry), Trientalis borealis (star-flower), and Medeola virginiana (Indian cumber-root) are typical. The successional status of this type seems variable, in some cases, especially on harsher sites, it appears relatively stable, in other cases it appears to be transitional. -

Tulip Tree Liriodendron Tulipifera
Tulip Tree Liriodendron tulipifera Liriodendron tulipifera is one of the tallest trees in the eastern United States. Its native range extends from Connecticut down the eastern seaboard to northern Florida, west to Louisiana, Tennessee, Indiana, and southern Illinois. In fact, it’s the state tree of Indiana, where enormous specimens can be found with trunks up to five feet in diameter and heights in excess of 100 feet. The tree is excurrent in growth habit, having a strong central leader. Mature tulip trees often lack branches for 50 or more feet from the ground, as if the main trunk were racing toward the sunlight. The leaf’s outline strongly resembles that of a tulip, hence not only the Latin name, but also the common names of tulip tree or tulip poplar. This species is also sometimes called yellow poplar, although it is not at all related to the trees of the Populus genus such as poplars, cottonwoods, or aspens. The tulip tree’s leaves turn a brilliant yellow in the fall, which makes the species an excellent landscape centerpiece. Early yellowing of the leaves in mid-summer, however, can be a sign of stress caused by drought or poor soil conditions. Liriodendron tulipifera is a member of the magnolia family, known for showy flowers. The tulip tree is no exception, boasting erect, cup-shaped flowers that are green-ish yellow with orange bases that bloom in May. The tree also has distinctive, flattened “duckbill” buds. This is a good winter identification tool, if there are branches low enough for the buds to be seen. -

Specializing in Rare and Unique Trees 2015 Catalogue
Whistling Gardens 698 Concession 3, Wilsonville, ON N0E 1Z0 Phone- 519-443-5773 Fax- 519-443-4141 Specializing in Rare and Unique Trees 2015 Catalogue Pot sizes: The number represents the size of the pot ie. #1= 1 gallon, #10 = 10 gallon #1 potted conifers are usually 3-5years old. #10 potted conifers dwarf conifers are between 10 and 15 years old #1 trees= usually seedlings #10 trees= can be several years old anywhere from 5 to 10' tall depending on species and variety. Please ask us on sizes and varieties you are not sure about. Many plants are limited to 1specimen. To reserve your plant(s) a 25% is required. Plants should be picked up by June 15th. Most plants arrive at the gardens by May 10th. Guarantee: We cannot control the weather (good or bad), rodents (big or small), pests (teenie, tiny), poor siting, soil types, lawnmovers, snowplows etc. Plants we carry are expected to grow within the parameters of normal weather conditons. All woody plant purchases are guaranteed from time of purchase to December 1st of current year Any plant not performing or dying in current season will be happily replaced or credited towards a new plant. Please email us if possible with any info needed about our plants. We do not have a phone in the garden centre and I'm rarely in the office. It is very helpful to copy and paste the botanical name of the plant into your Google browser, in most cases, a d Order Item Size Price Description of Pot European White Fir Zone 5 Abies alba Barabit's Spreader 25-30cm #3 $100.00 Abies alba Barabit's Spreader 40cm -

Supplementary Materials Neodiprion Sawflies
Supplementary Materials Neodiprion sawflies: life history description and utility as a model for parent-offspring conflict To provide context for our comparative analysis of Neodiprion clutch-size traits, we provide relevant life history details (reviewed in Coppel and Benjamin 1965; Knerer and Atwood 1973; Wilson et al. 1992; Knerer 1993). Adult females emerge from cocoons with a full complement of mature eggs, find a suitable host, and attract males via a powerful pheromone. Shortly after mating, females use their saw-like ovipositors to embed their eggs within pine needles. While females of some species tend to lay their full complement of eggs on a single branch terminus, females of other species seek out multiple branches or trees for oviposition. Overall, female oviposition behavior is highly species-specific and, in some cases, diagnostic (Ghent 1959). Adult Neodiprion are non-feeding and short-lived (~2-4 days), dying soon after mating and oviposition. After hatching from eggs, Neodiprion larvae of many species form feeding aggregations that remain intact to varying degrees across 4-7 feeding instars, depending on the sex and the species. As larvae defoliate pine branches, they migrate to new branches and sometimes to new host trees (Benjamin 1955, Smirnoff 1960). During these migrations, colonies may undergo fission and fusion events (Codella and Raffa 1993, Codella and Raffa 1995, Costa and Loque 2001). Thus, while initial colony size corresponds closely to egg-clutch size, larvae are highly mobile and their dispersal behavior has the potential to substantially alter colony size (Codella and Raffa 1995). Beyond having a variable and well-documented natural history, Neodiprion provides an excellent test case for examining coevolution of female egg-laying and larval grouping behaviors because, as is likely the case for many insects, feeding in groups could confer both costs and benefits to the larvae (Codella and Raffa 1995, Heitland and Pschorn-Walcher 1993). -

Deer-Resistant Landscape Plants This List of Deer-Resistant Landscape Plants Was Compiled from a Variety of Sources
Deer-Resistant Landscape Plants This list of deer-resistant landscape plants was compiled from a variety of sources. The definition of a deer-resistant plant is one that may be occasionally browsed, but not devoured. If deer are hungry enough or there’s a limited amount of food available, they will eat almost anything. Perennials, Herbs & Bulbs Achillea Yarrow Lavandula augustifolia Lavender Adiatum pedatum Maiden Hair Fern Liatris Gayfeather Agastache cana Agastache Lychnis chalcedonica Maltese Cross Ajuga reptans Bugleweed Matteuccia Ostrich Fern Alchemilla Lady’s Mantle Mentha Mint Allium spp. Flowering Onion Miscanthus Silver Grass Arabis Rock Cress Myosotis sylvatica Forget-Me-Not Armeria maritime Sea Thrift Narcissus Daffodils & Narcissus Artemisia Wormwood Nepeta Catmint Asarum europaeum Wild Ginger Oenothera Evening Primrose Asclepias tuberosa Butterfly Weed Paeonia Peony Bergenia Bergenia Papaver orientale Oriental Poppy Calamagrostis Reed Grass Pennisetum orientale Oriental Fountain Grass Cerastium Snow-In-Summer Perovskia Russian Sage Cimcifuga Bugbane Physostegia Obedient Plant Convallaria Lily-of-the-Valley Platycodon Balloon Flower Corydalis lutea Gold Bleeding Heart Polygonatum Solomon’s Seal Dicentra Bleeding Heart Pulmonaria Lungwort Digitalis Foxglove Rudbeckia Black Eyed Susan Echinops Globe Thistle Saponaria Soapwort Festuca ovinia Blue Fescue Salvia Sage Galanthus nivalis Snowdrop Sedum Stonecrop Helleborus Hellebores Solidago Goldenrod Iris sibirica & ensata Japanese & Siberian Iris Stachys byzantina Lamb’s Ear Lamium -

Upper Canopy Collection and Identification of Grapevines (Vitis) from Selected Forests in the Southeastern United States Sydney E
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Papers in Plant Pathology Plant Pathology Department 2010 Upper Canopy Collection and Identification of Grapevines (Vitis) from Selected Forests in the Southeastern United States Sydney E. Everhart University of Nebraska-Lincoln, [email protected] Follow this and additional works at: http://digitalcommons.unl.edu/plantpathpapers Part of the Other Plant Sciences Commons, Plant Biology Commons, and the Plant Pathology Commons Everhart, Sydney E., "Upper Canopy Collection and Identification of Grapevines (Vitis) from Selected Forests in the Southeastern United States" (2010). Papers in Plant Pathology. 364. http://digitalcommons.unl.edu/plantpathpapers/364 This Article is brought to you for free and open access by the Plant Pathology Department at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Papers in Plant Pathology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. CASTANEA 75(1): 141–149. MARCH 2010 Upper Canopy Collection and Identification of Grapevines (Vitis) from Selected Forests in the Southeastern United States Sydney E. Everhart* Department of Biology and Earth Science, University of Central Missouri, Warrensburg, Missouri 64093 ABSTRACT Woody grapevines (Vitis spp.) are common in the deciduous forests of the southeastern United States. Their growth habit makes leaf collection challenging and polymorphic leaves make identification of species difficult. Mature grapevines can grow up to 48 cm in diameter at breast height and reach the upper canopy of trees more than 35 m in height. Leaf morphology is the most readily available character used for species identification. However, most mature grapevines do not produce leaves below the upper canopy and if they do, these leaves are morphologically indistinguishable from other species. -
Trees & Conifers
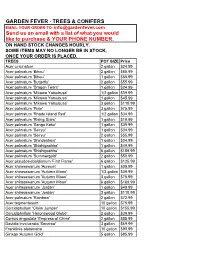
GARDEN FEVER - TREES & CONIFERS EMAIL YOUR ORDER TO: [email protected] Send us an email with a list of what you would like to purchase & YOUR PHONE NUMBER. ON HAND STOCK CHANGES HOURLY. SOME ITEMS MAY NO LONGER BE IN STOCK, ONCE YOUR ORDER IS PLACED. TREES POT SIZE Price Acer ciricinatum 2 gallon $24.99 Acer palmatum 'Bihou' 2 gallon $85.99 Acer palmatum 'Bihou' 1 gallon $55.99 Acer palmatum 'Butterfly' 2 gallon $55.99 Acer palmatum 'Dragon Tears' 1 gallon $24.99 Acer palmatum 'Mikawa Yatsubusa' 1/2 gallon $39.99 Acer palmatum 'Mikawa Yatsubusa' 1 gallon $45.99 Acer palmatum 'Mikawa Yatsubusa' 3 gallon $110.99 Acer palmatum 'Pixie' 3 gallon $75.99 Acer palmatum 'Rhode Island Red' 1/2 gallon $34.99 Acer palmatum 'Rising Stars' 1 gallon $19.99 Acer palmatum 'Sango Kaku' 1 gallon $39.99 Acer palmatum 'Seiryu' 1 gallon $34.99 Acer palmatum 'Seiryu' 2 gallon $55.99 Acer palmatum 'Shindeshojo' 1 gallon $34.99 Acer palmatum 'Shishigashira' 1 gallon $49.99 Acer palmatum 'Shishigashira' 6 gallon $159.99 Acer palmatum 'Summergold' 2 gallon $59.99 Acer pseudosieboldianum 'First Flame' 6 gallon $125.99 Acer shirasawanum 'Aureum' 1 gallon $39.99 Acer shirasawanum 'Autumn Moon' 1/2 gallon $39.99 Acer shirasawanum 'Autumn Moon' 3 gallon $75.99 Acer shirasawanum 'Autumn Moon' 6 gallon $169.99 Acer shirasawanum 'Jordan' 1 gallon $49.99 Acer shirasawanum 'Jordan' 3 gallon $110.99 Acer palmatum 'Rainbow' 2 gallon $72.99 Acer tegmentosum 10 gallon $75.99 Cercidiphyllum 'Claim Jumper' 10 gallon $155.99 Cercidiphyllum 'Heronswood Globe' 2 gallon $39.99 Cornus