Effect of Carvedilol on Heart Rate Response to Cardiopulmonary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Summary of Product Characteristics
Health Products Regulatory Authority Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Cardisure 3.5 mg/ml Oral Solution for Dogs 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains Active substance: Pimobendan 3.5 mg Excipients: Benzyl alcohol (E1519) 1.0 mg For the full list of excipients, see section 6.1. 3 PHARMACEUTICAL FORM Oral Solution. Clear, colourless, semi-viscous liquid. 4 CLINICAL PARTICULARS 4.1 Target Species Dogs. 4.2 Indications for use, specifying the target species For the treatment of canine congestive heart failure originating from valvular insufficiency (mitral and/or tricuspid regurgitation) or dilated cardiomyopathy. 4.3 Contraindications Do not use in cases of hypertrophic cardiomyopathies or clinical conditions where an augmentation of cardiac output is not possible for functional or anatomical reasons (e.g. aortic stenosis). Do not use in dogs with severe impairment of liver function, as pimobendan is metabolised mainly via the liver. Do not use in cases of known hypersensitivity to the active substance or to any of the excipients. 4.4 Special warnings for each target species None known. 4.5 Special precautions for use Special precautions for use in animals The blood glucose should be tested regularly during treatment in dogs with existing diabetes mellitus. Monitoring of cardiac function and morphology is recommended in animals treated with pimobendan (See also section 4.6). Special precautions to be taken by the person administering the veterinary medicinal product to animals Accidental ingestion, especially by a child, may lead to the occurrence of tachycardia, orthostatic hypotension, flushing of the face and headaches. -

Dynamic Analysis of Cardiovascular Drugs Data
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2014, 6(5):1517-1520 ISSN : 0975-7384 Research Article CODEN(USA) : JCPRC5 Dynamic analysis of cardiovascular drugs data Humei Yang and SunShi The Affiliated Hospital of Qingdao University Medical College , China _____________________________________________________________________________________________ ABSTRACT Cardiovascular disease is an important disease which is a serious hazard to human health, not only that, but the cause is still quite complicated. Therefore, it is very important to carry out research work of cardiovascular drugs in the medical profession. In this article, the money amount sequence method and DDD number sequence method were used to statistically analyze the data of cardiovascular drugs which were purchased by patients in a hospital in Shanghai in 2010-2013 year. The data of cardiovascular drugs second-class hospitals and upper-class hospitals in Shanghai included the drug varieties, prescription medication, the amount of money, which were statistically analyzed in order to provide recommendations for future cardiovascular medication guide. Through this research, we have come to the result that the money of cardiovascular drugs accounts greater of the amount of all of the drugs in the second-class hospitals than that in the upper-class hospitals, and cardiovascular medication amounts, prescriptions, etc of the second-class hospitals and upper-class hospitals had increased than before. Calcium antagonists and angiotensin ⅱ receptor antagonist drugs led occupation, as well as a small amount of varieties of drugs had gone into the top 10 amounts .As for cardiovascular drugs, foreign markets occupy a major market. That is to say that between different levels of hospitals there is some difference in cardiovascular drug use, but the drugs with good effects are already the first choice. -

SNF Mobility Model: ICD-10 HCC Crosswalk, V. 3.0.1
The mapping below corresponds to NQF #2634 and NQF #2636. HCC # ICD-10 Code ICD-10 Code Category This is a filter ceThis is a filter cellThis is a filter cell 3 A0101 Typhoid meningitis 3 A0221 Salmonella meningitis 3 A066 Amebic brain abscess 3 A170 Tuberculous meningitis 3 A171 Meningeal tuberculoma 3 A1781 Tuberculoma of brain and spinal cord 3 A1782 Tuberculous meningoencephalitis 3 A1783 Tuberculous neuritis 3 A1789 Other tuberculosis of nervous system 3 A179 Tuberculosis of nervous system, unspecified 3 A203 Plague meningitis 3 A2781 Aseptic meningitis in leptospirosis 3 A3211 Listerial meningitis 3 A3212 Listerial meningoencephalitis 3 A34 Obstetrical tetanus 3 A35 Other tetanus 3 A390 Meningococcal meningitis 3 A3981 Meningococcal encephalitis 3 A4281 Actinomycotic meningitis 3 A4282 Actinomycotic encephalitis 3 A5040 Late congenital neurosyphilis, unspecified 3 A5041 Late congenital syphilitic meningitis 3 A5042 Late congenital syphilitic encephalitis 3 A5043 Late congenital syphilitic polyneuropathy 3 A5044 Late congenital syphilitic optic nerve atrophy 3 A5045 Juvenile general paresis 3 A5049 Other late congenital neurosyphilis 3 A5141 Secondary syphilitic meningitis 3 A5210 Symptomatic neurosyphilis, unspecified 3 A5211 Tabes dorsalis 3 A5212 Other cerebrospinal syphilis 3 A5213 Late syphilitic meningitis 3 A5214 Late syphilitic encephalitis 3 A5215 Late syphilitic neuropathy 3 A5216 Charcot's arthropathy (tabetic) 3 A5217 General paresis 3 A5219 Other symptomatic neurosyphilis 3 A522 Asymptomatic neurosyphilis 3 A523 Neurosyphilis, -

Receptores Β-Adrenérgicos No Sistema Cardiovascular
Medicina, Ribeirão Preto, X SIMPÓSIO BRASILEIRO DE FISIOLOGIA CARDIOVASCULAR 39 (1): 3-12, jan./mar. 2006 Capítulo I RECEPTORES β-ADRENÉRGICOS NO SISTEMA CARDIOVASCULAR β-ADRENOCEPTORS FUNCTION IN THE CARDIOVASCULAR SYSTEM Mário A Neto1, Ricardo R Rascado1, Lusiane M Bendhack2 1Pós-Graduandos (Doutorado). Faculdade de Medicina de Ribeirão Preto - USP. 2Docente. Departamento de Física e Química. Faculdade de Ciências Farmacêuticas de Ribeirão Preto - USP. CORRESPONDÊNCIA: Profa. Dra. Lusiane M. Bendhack. Laboratório de Farmacologia. Faculdade de Ciências Farmacêuticas de Ribeirão Preto - USP Av. do Café s/nº - 14040-903 Ribeirão Preto - SP Fax: 16-36024880 . e-mail: [email protected] Neto MA, Rascado RR, Bendhack LM. Receptores β−adrenérgicos no sistema cardiovascular. Medicina (Ribeirão Preto) 2006; 39 (1): 3-12. Resumo: Os receptores β-adrenérgicos (β-AR) integram um sistema proteico ternário: β-AR, proteína G de acoplamento e enzimas como a adenilato-ciclase (AC) que produz o 3´-5´ monofosfato de adenosina (AMPc). O principal mecanismo de ação do AMPc é a ativação da proteína quinase A (PKA), capaz de fosforilar inúmeros substratos. Em células endoteliais, a ativação dos β-AR promove o aumento dos níveis citoplasmáticos de Ca+2 favorecendo a ligação do Ca+2 com a calmodulina e deste complexo com a enzima óxido nítrico sintase endotelial (eNOS), resultando na produção de NO. A ativação β-AR no músculo liso vascular e conseqüente ativação da PKA reduz a concentração citoplasmática de Ca+2 e a sua afinidade pela calmodulina, resultando no relaxamento vascular. Os receptores β-AR podem ser agrupados em β-AR clássi- cos (β e β ) e β-AR atípicos (β e β ). -

Controlled Substance Monitoring and Drugs of Abuse Testing Local Coverage Determination
Controlled Substance Monitoring and Drugs of Abuse Testing Local Coverage Determination CPT Codes: Code Description 80305 Drug test(s), presumptive, any number of drug classes, any number of devices or procedures (eg, immunoassay); capable of being read by direct optical observation only (eg, dipsticks, cups, cards, cartridges) includes sample validation when performed, per date of service 80306 Drug test(s), presumptive, any number of drug classes, any number of devices or procedures (eg, immunoassay); read by instrument assisted direct optical observation (eg, dipsticks, cups, cards, cartridges), includes sample validation when performed, per date of service 80307 Drug test(s), presumptive, any number of drug classes, any number of devices or procedures, by instrument chemistry analyzers (eg, utilizing immunoassay [eg, eia, elisa, emit, fpia, ia, kims, ria]), chromatography (eg, gc, hplc), and mass spectrometry either with or without chromatography, (eg, dart, desi, gc-ms, gc-ms/ms, lc-ms, lc-ms/ms, ldtd, maldi, tof) includes sample validation when performed, per date of service G0480 Drug test(s), definitive, utilizing drug identification methods able to identify individual drugs and distinguish between structural isomers (but not necessarily stereoisomers), including, but not limited to gc/ms (any type, single or tandem) and lc/ms (any type, single or tandem and excluding immunoassays (e.g., ia, eia, elisa, emit, fpia) and enzymatic methods (e.g., alcohol dehydrogenase)); qualitative or quantitative, all sources(s), includes specimen -

Malta Medicines List April 08
Defined Daily Doses Pharmacological Dispensing Active Ingredients Trade Name Dosage strength Dosage form ATC Code Comments (WHO) Classification Class Glucobay 50 50mg Alpha Glucosidase Inhibitor - Blood Acarbose Tablet 300mg A10BF01 PoM Glucose Lowering Glucobay 100 100mg Medicine Rantudil® Forte 60mg Capsule hard Anti-inflammatory and Acemetacine 0.12g anti rheumatic, non M01AB11 PoM steroidal Rantudil® Retard 90mg Slow release capsule Carbonic Anhydrase Inhibitor - Acetazolamide Diamox 250mg Tablet 750mg S01EC01 PoM Antiglaucoma Preparation Parasympatho- Powder and solvent for solution for mimetic - Acetylcholine Chloride Miovisin® 10mg/ml Refer to PIL S01EB09 PoM eye irrigation Antiglaucoma Preparation Acetylcysteine 200mg/ml Concentrate for solution for Acetylcysteine 200mg/ml Refer to PIL Antidote PoM Injection injection V03AB23 Zovirax™ Suspension 200mg/5ml Oral suspension Aciclovir Medovir 200 200mg Tablet Virucid 200 Zovirax® 200mg Dispersible film-coated tablets 4g Antiviral J05AB01 PoM Zovirax® 800mg Aciclovir Medovir 800 800mg Tablet Aciclovir Virucid 800 Virucid 400 400mg Tablet Aciclovir Merck 250mg Powder for solution for inj Immunovir® Zovirax® Cream PoM PoM Numark Cold Sore Cream 5% w/w (5g/100g)Cream Refer to PIL Antiviral D06BB03 Vitasorb Cold Sore OTC Cream Medovir PoM Neotigason® 10mg Acitretin Capsule 35mg Retinoid - Antipsoriatic D05BB02 PoM Neotigason® 25mg Acrivastine Benadryl® Allergy Relief 8mg Capsule 24mg Antihistamine R06AX18 OTC Carbomix 81.3%w/w Granules for oral suspension Antidiarrhoeal and Activated Charcoal -
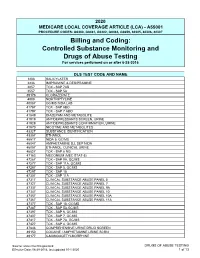
Controlled Substance Monitoring and Drugs of Abuse Testing for Services Performed on Or After 6-28-2016
2020 MEDICARE LOCAL COVERAGE ARTICLE (LCA) - A55001 PROCEDURE CODES: G0480, G0481, G0482, G0483, G0659, 80305, 80306, 80307 Billing and Coding: Controlled Substance Monitoring and Drugs of Abuse Testing For services performed on or after 6-28-2016 DLS TEST CODE AND NAME 1203 SALICYLATES 2236 IMIPRAMINE & DESIPRAMINE 2857 TOX - SAP 7AB 3557 TOX - SAP 5A 39176 CLORAZEPATE 4008 NORTRIPTYLINE 40087 GC/MS NIDA LAB 41787 TOX - SAP 6BO 41797 TOX - SAP 7 ABO 41849 DIAZEPAM AND METABOLITE 41919 ANTIDEPRESSANTS SCREEN, URINE 41929 ANTIDEPRESSANTS CONFIRMATION, URINE 41970 NICOTINE AND METABOLITES 43327 SUBSTANCE IDENTIFICATION 43357 ETHANOL 45517 NIDA 5, GC/MS 46047 AMPHETAMINE D,L SEP NIDA 46057 ETHANOL, CLINICAL URINE 46827 TOX - SAP 6 NG 47163 MECONIUM (MEC STAT-5) 47267 TOX - SAP 9A, GC/MS 47277 TOX - SAP 11A, GC/MS 47287 TOX - SAP 5, GC/MS 47297 TOX - SAP 10 47307 TOX - SAP 11A 47317 CLINICAL SUBSTANCE ABUSE PANEL 5 47327 CLINICAL SUBSTANCE ABUSE PANEL 7 47337 CLINICAL SUBSTANCE ABUSE PANEL 9A 47347 CLINICAL SUBSTANCE ABUSE PANEL 10 47357 CLINICAL SUBSTANCE ABUSE PANEL 10A 47367 CLINICAL SUBSTANCE ABUSE PANEL 11A 47377 TOX - SAP 10, GC/MS 47387 TOX - SAP 5A,GC/MS 47397 TOX - SAP 6, GC/MS 47407 TOX - SAP 7, GC/MS 47417 TOX - SAP 7A, GC/MS 47427 TOX - SAP 4, GC/MS 47846 COMPREHENSIVE URINE DRUG SCREEN 49150 COCAINE / AMPHETAMINE URINE SCRN 49177 6-MONOACETYLMORPHINE Source: www.cms.hhs.gov/mcd DRUGS OF ABUSE TESTING Effective Date: 06-28-2016, last updated 10-1-2020 1 of 13 49237 AMPHETAMINES, CLINICAL UR 49247 BARBITURATES, CLINICAL URINE 49257 -

UWA Research Publication
UWA Research Publication Price, S., Holman, D. A., Sanfilippo, F., & Emery, J. (2013). Use of case–time–control design in pharmacovigilance applications: exploration with high-risk medications and unplanned hospital admissions in the Western Australian elderly. Pharmacoepidemiology and Drug Safety, 22(11), 1159-1170. Copyright © 2013 John Wiley & Sons, Ltd. This is the peer reviewed version of the following article: Price, S., Holman, D. A., Sanfilippo, F., & Emery, J. (2013). Use of case–time–control design in pharmacovigilance applications: exploration with high-risk medications and unplanned hospital admissions in the Western Australian elderly. Pharmacoepidemiology and Drug Safety, 22(11), 1159-1170., which has been published in final form at http://dx.doi.org/10.1002/pds.3469. This article may be used for non-commercial purposes in accordance With Wiley Terms and Conditions for self-archiving' This version was made available in the UWA Research Repository on 23 April 2015 in compliance with the publisher’s policies on archiving in institutional repositories. Use of the article is subject to copyright law. Use of case-time-control design in pharmacovigilance applications: exploration with high risk medications and unplanned hospital admissions in the Western Australian elderly Running head: High risk drugs and hospital admissions in elderly Sylvie D Price1, C D’Arcy J Holman1, Frank M Sanfilippo1 and Jon D Emery2 1 School of Population Health, The University of Western Australia, Perth, Australia 2 School of Primary, Aboriginal and Rural Health Care, The University of Western Australia, Perth, Australia Corresponding author: Sylvie Price, School of Population Health (M431), The University of Western Australia, 35 Stirling Highway, CRAWLEY WA 6009, Australia; Tel. -

Adult Intravenous Medications
Revised 9/08 ADULT INTRAVENOUS MEDICATIONS STANDARD AND MAXIMUM ALLOWABLE CONCENTRATIONS, GUIDELINES FOR CONTINUOUS OR TITRATED INFUSIONS MEDICATION STANDARD MAXIMUM CONC./ DOSING MONITORING/COMMENTS ADMIXTURE INFUSION INSTRUCTIONS Adenosine 6 mg/2 mL vial Give undiluted directly into 6 mg initially. If SVT not ECG, heart rate, blood pressure (Adenocard®) (3 mg/mL) given undiluted vein over 1-2 seconds. resolved in 1-2 minutes, may Administer as proximal as follow with 12 mg dose. If Extremely short half life: possible to trunk (i.e., not in not resolved in 1-2 minutes, < 10 seconds Slows conduction time through the AV node, interrupting the re-entry lower arm, hand, lower leg, may follow with an Not effective for converting A. pathways through the AV node, or foot). If administered additional 12 mg dose. flutter, A. fib, or ventricular restoring normal sinus rhythm. through IV line, administer tachycardia. as close to pts heart as Contraindicated if symptomatic Onset of action: immediate Duration: seconds possible. NS flush must be bradycardia, sick sinus given rapidly, immediately syndrome, 2nd or 3rd degree AV following injection of block (unless pt. has functioning adenosine pacemaker) Amiodarone Load: Dilute 150 mg (3mL) in Peripheral line: Up to 2 Load: 150 mg/100 mL over Telemetry monitoring, BP (Cordarone®) 100 mL D5W (1.5 mg/mL) mg/mL 10 minutes. (hypotension occurs frequently (PVC bag suitable for loading (Not to eXceed 30 mg/mL) with initial rates), HR Antiarrhythmic agent that dose) (Concentrations over 2 (arrhythmias: AV block, depresses conduction velocity, mg/mL administered for THEN bradycardia, VT/VF, torsades de slows AV node conduction, raises Maintenance infusion: longer than 1 hour must be pointes), electrolytes the threshold for VF, and eXhibits Dilute 900 mg (18 mL) in infused via central line) Infusion: 1 mg/min for 6 some α and β blockade activity. -

Summary of Product Characteristics 1. Name Of
Revised: June 2016 AN: 00061/2016 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Boehringer Pimobendan 0.75 mg/ml Solution for Injection for Dogs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One ml contains: Active substance: Pimobendan 0.75 mg Excipients: For a full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Clear colourless solution for injection 4. CLINICAL PARTICULARS 4.1 Target species Dogs 4.2 Indications for use, specifying the target species To initiate treatment of canine congestive heart failure originating from valvular insufficiency (mitral and/or tricuspid regurgitation) or dilated cardiomyopathy. 4.3 Contraindications The product should not be used in cases of hypertrophic cardiomyopathies or clinical conditions where an augmentation of cardiac output is not possible for functional or anatomical reasons (e.g. aortic stenosis). See also section 4.7. 4.4 Special warnings None 4.5 Special precautions for use Page 1 of 5 Revised: June 2016 AN: 00061/2016 Special precautions for use in animals In the event of accidental subcutaneous injection, temporary swelling and mild resorptive inflammatory reactions may occur at or below the injection site. For single administration only. Special precautions to be taken by the person administering the veterinary medicinal product to animals In case of accidental self-injection, seek medical advice immediately and show the package leaflet or the label to the physician. 4.6 Adverse reactions (frequency and seriousness) A moderate positive chronotropic effect and vomiting may occur in rare cases. In rare cases transient diarrhoea, anorexia or lethargy have been observed. 4.7 Use during pregnancy, lactation or lay In studies with rats and rabbits pimobendan had no effect on fertility. -

Formulary April
Standard Formulary MedPerform Medium April, 2021 Copyright © 2020 MedImpact Healthcare Systems, Inc. All rights reserved. This document is confidential and proprietary to MedImpact and contains material MedImpact may consider Trade Secrets. This document is intended for specified use by Business Partners of MedImpact under permission by MedImpact and may not otherwise be used, reproduced, transmitted, published, or disclosed to others without prior written authorization. MedImpact maintains the sole and exclusive ownership, right, title, and interest in and to this document. MedPerform Medium Formulary What is the standard formulary? The MedImpact formulary is a list of covered drugs selected by physician and pharmacist subject matter experts who collaboratively support MedImpact’s Pharmacy and Therapeutics (P&T) Committee. The plan will cover drugs listed in the formulary as long as the drug is indicated for the clinical condition, is prescribed in the appropriate manner, the prescription is filled at a participating network pharmacy, and other plan rules are followed. For more information on how to fill your prescriptions, please review your Evidence of Coverage. Can the Formulary (drug list) change? Drugs may be added or deleted from the formulary during the year. If a drug is removed from the formulary, [or] adds prior authorization, quantity limits and/or step therapy restrictions on a drug or moves a drug to a higher cost-sharing tier], the plan will notify affected members of the change before the change becomes effective. If the Food and Drug Administration (FDA) deems a drug on the formulary to be unsafe or the drug’s manufacturer removes the drug from the market, the plan will immediately remove the drug from the formulary. -

Quality Use of Cardiovascular Medicines (169K PDF)
5 Quality use of cardiovascular medicines This chapter presents information on several aspects of the appropriate use of medicines for cardiovascular disease for which data are available and describes national initiatives aimed at achieving quality use of these medicines where deficiencies have been identified. 5.1 Medicines prescribed for cardiovascular disease Data on cardiovascular medicines that GPs prescribed or supplied for specific cardiovascular conditions and risk factors (hypertension, lipid disorders and diabetes) were sourced from the BEACH study for the period April 2000–March 2006. For information on this study and details of the method used to analyse the data, see the Appendix. Over the period 2000–2006, GPs prescribed or supplied medicines to manage most cardiovascular problems (Table 5). Table 5: Medicines prescribed or supplied by GPs for specific problems, 2000–06 Problem managed Medicine rate per 100 problems (95%CI) Arrhythmia 81.1 (78.1–84.2) Diabetes 72.3 (70.5–74.1) Heart failure 118.6 (114.2–123.0) Hypertension 96.5 (95.2–97.9) Ischaemic heart disease 116.6 (113.1–120.1) Lipid disorder 65.4 (64.4–66.5) Peripheral vascular disease 37.4 (32.7–42.1) Stroke 58.7 (53.3–64.1) Source: BEACH study (unpublished). Hypertension is the most common problem managed in general practice, at 6.5% of all problems in 2005–06. Between 2000–01 and 2005–06 the rate of management of hypertension by GPs increased (from 8.6 to 9.4 hypertension problems managed per 100 encounters). In 2005–06, agents acting on the renin–angiotensin system were by far the most popular medicines prescribed or supplied for hypertension (53.4 per 100 problems), followed by calcium-channel blockers (18.9 per 100 problems), beta-blocking agents (11.9 per 100 problems), and diuretics (7.4 per 100 problems), while antihypertensives were seldom prescribed (1.7 per 100 problems) (Figure 9 and Table e1).