Anisoptera: Petaluridae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Using Specimens from the Past to Understand the Living World Through Digitization
Using specimens from the past to understand the living world through digitization Drs. Jessica L. Ware, William Kuhn, Dirk Gassmann and John Abbott Rutgers University, Newark Dragonflies: Order Odonata Suborders Anisoptera (unequal wings): Dragonflies ~3000 species Anisozygoptera ~3 species Zygoptera: Damselflies ~3000 species Perchers, Fliers, Migrators, & Homebodies Dragonfly flight Wing venation affects wing camber, lift, and ultimately flight patterns Dragonfly flight Stiffness varies along length of the wing with vein density and thickness Dragonfly flight Certain wing traits are correlated with specific flight styles Dragonfly collections: invaluable treasures Collection name # spp. #specimens Florida State Collection 2728 150K Ware Lab Collection 373 4K Smithsonian Collection 253 200K M.L. May Collection 300 10K Dragonfly collections: invaluable treasures Harness information in collections Targeted Odonata Wing Digitization (TOWD) project TOWD project scanning protocol TOWD project scanning protocol TOWD project TOWD project TOWD project 10 10 TOWD project More information at: https://willkuhn.github.io/towd/ Aspect ratios: How elongate is the wing compared to its overall area? • High Aspect ratio Low Aspect ratio Long narrow wings, Wing Short broad wings, optimized for: Wing optimized long-distance flight for: Maneuverability, turning High Aspect Low Aspect Ratio Ratio More data on aspect ratios, better interpretations? 2007: Hand measured forewings of 85 specimens, 7 months work More data on apsect ratios, better interpretations? 2007: Hand measured forewings of 85 specimens, 7 months 2019: Odomatic measurements for 206 work specimens, 2-3 minutes of work! Are there differences in aspect ratios? Perchers have significantly lower aspect ratios than fliers. The p-value is .001819. The result is significant at p < .05. -

Gleaning on Coreidae (Heteroptera) by Tachopteryx Thoreyi (Odonata: Petaluridae)
The Great Lakes Entomologist Volume 31 Numbers 3 & 4 - Fall/Winter 1998 Numbers 3 & Article 12 4 - Fall/Winter 1998 October 1998 Gleaning on Coreidae (Heteroptera) by Tachopteryx Thoreyi (Odonata: Petaluridae) R. D. Waltz IDNR Follow this and additional works at: https://scholar.valpo.edu/tgle Part of the Entomology Commons Recommended Citation Waltz, R. D. 1998. "Gleaning on Coreidae (Heteroptera) by Tachopteryx Thoreyi (Odonata: Petaluridae)," The Great Lakes Entomologist, vol 31 (3) Available at: https://scholar.valpo.edu/tgle/vol31/iss3/12 This Peer-Review Article is brought to you for free and open access by the Department of Biology at ValpoScholar. It has been accepted for inclusion in The Great Lakes Entomologist by an authorized administrator of ValpoScholar. For more information, please contact a ValpoScholar staff member at [email protected]. Waltz: Gleaning on Coreidae (Heteroptera) by <i>Tachopteryx Thoreyi</i> 1998 THE GREAT LAKES ENTOMOLOGIST 209 GLEANING ON COREIDAE (HETEROPTERA) BY TACHOPTERYX THOREYI (ODONATA: PETALURIDAEl R.D. Woltz 1 Tachopteryx thoreyi (Hagen in Selys) is an uncommon or rare, fen and seep dwelling dragonfly noted for its large size, restricted habitat require ments, and characteristic habit of perching on tree trunks. While performing insect sampling and habitat assessments in a fen at Mounds State Park, Anderson, Indiana, I observed a male T. thoreyi land on a tree trunk and capture an insect on the bark of the tree, 1 June 1998, at approximately 1430 hr. Upon approaching and capturing the dragonfly, I found that it had captured and held in its grasp a large leaf-footed bug, prob ably Acanthocephala terminalis (Dallas) (Heteroptera: Coreidae). -

André Nel Sixtieth Anniversary Festschrift
Palaeoentomology 002 (6): 534–555 ISSN 2624-2826 (print edition) https://www.mapress.com/j/pe/ PALAEOENTOMOLOGY PE Copyright © 2019 Magnolia Press Editorial ISSN 2624-2834 (online edition) https://doi.org/10.11646/palaeoentomology.2.6.1 http://zoobank.org/urn:lsid:zoobank.org:pub:25D35BD3-0C86-4BD6-B350-C98CA499A9B4 André Nel sixtieth anniversary Festschrift DANY AZAR1, 2, ROMAIN GARROUSTE3 & ANTONIO ARILLO4 1Lebanese University, Faculty of Sciences II, Department of Natural Sciences, P.O. Box: 26110217, Fanar, Matn, Lebanon. Email: [email protected] 2State Key Laboratory of Palaeobiology and Stratigraphy, Center for Excellence in Life and Paleoenvironment, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing 210008, China. 3Institut de Systématique, Évolution, Biodiversité, ISYEB-UMR 7205-CNRS, MNHN, UPMC, EPHE, Muséum national d’Histoire naturelle, Sorbonne Universités, 57 rue Cuvier, CP 50, Entomologie, F-75005, Paris, France. 4Departamento de Biodiversidad, Ecología y Evolución, Facultad de Biología, Universidad Complutense, Madrid, Spain. FIGURE 1. Portrait of André Nel. During the last “International Congress on Fossil Insects, mainly by our esteemed Russian colleagues, and where Arthropods and Amber” held this year in the Dominican several of our members in the IPS contributed in edited volumes honoring some of our great scientists. Republic, we unanimously agreed—in the International This issue is a Festschrift to celebrate the 60th Palaeoentomological Society (IPS)—to honor our great birthday of Professor André Nel (from the ‘Muséum colleagues who have given us and the science (and still) national d’Histoire naturelle’, Paris) and constitutes significant knowledge on the evolution of fossil insects a tribute to him for his great ongoing, prolific and his and terrestrial arthropods over the years. -

An Overview of Molecular Odonate Studies, and Our Evolutionary Understanding of Dragonfly and Damselfly (Insecta: Odonata) Behavior
International Journal of Odonatology Vol. 14, No. 2, June 2011, 137–147 Dragons fly, biologists classify: an overview of molecular odonate studies, and our evolutionary understanding of dragonfly and damselfly (Insecta: Odonata) behavior Elizabeth F. Ballare* and Jessica L. Ware Department of Biological Sciences, Rutgers, The State University of New Jersey, 195 University Ave., Boyden Hall, Newark, NJ, 07102, USA (Received 18 November 2010; final version received 3 April 2011) Among insects, perhaps the most appreciated are those that are esthetically pleasing: few capture the interest of the public as much as vibrantly colored dragonflies and damselflies (Insecta: Odonata). These remarkable insects are also extensively studied. Here, we review the history of odonate systematics, with an emphasis on discrepancies among studies. Over the past century, relationships among Odonata have been reinterpreted many times, using a variety of data from wing vein morphology to DNA. Despite years of study, there has been little consensus about odonate taxonomy. In this review, we compare odonate molecular phylogenetic studies with respect to gene and model selection, optimality criterion, and dataset completeness. These differences are discussed in relation to the evolution of dragonfly behavior. Keywords: Odonata; mitochondrion; nuclear; phylogeny; systematic; dragonfly; damselfly Introduction Why study Odonata? The order Odonata comprises three suborders: Anisozygoptera, Anisoptera, and Zygoptera. There are approximately 6000 species of Odonata described worldwide (Ardila-Garcia & Gregory, 2009). Of the three suborders Anisoptera and Zygoptera are by far the most commonly observed and collected, because there are only two known species of Anisozygoptera under the genus Epiophlebia. All odonate nymphs are aquatic, with a few rare exceptions such as the semi-aquatic Pseudocordulia (Watson, 1983), and adults are usually found near freshwater ponds, marshes, rivers (von Ellenrieder, 2010), streams, and lakes (although some species occur in areas of mild salinity; Corbet, 1999). -

Phylogenetic Analysis of the Insect Order Odonata Using 28S and 16S Rdna Sequences: a Comparison Between Data Sets with Different Evolutionary Rates
Entomological Science (2006) 9, 55–66 doi:10.1111/j.1479-8298.2006.00154.x ORIGINAL ARTICLE Phylogenetic analysis of the insect order Odonata using 28S and 16S rDNA sequences: a comparison between data sets with different evolutionary rates Eisuke HASEGAWA1 and Eiiti KASUYA2 1Laboratory of Animal Ecology, Department of Ecology and Systematics, Graduate School of Agriculture, Hokkaido University, Sapporo and 2Department of Biology, Faculty of Sciences, Kyushu University, Fukuoka, Japan Abstract Molecular phylogenetic analyses were conducted for the insect order Odonata with a focus on testing the effectiveness of a slowly evolving gene to resolve deep branching and also to examine: (i) the monophyly of damselflies (the suborder Zygoptera); and (ii) the phylogenetic position of the relict dragonfly Epiophlebia superstes. Two independent molecular sources were used to reconstruct phylogeny: the 16S rRNA gene on the mitochondrial genome and the 28S rRNA gene on the nuclear genome. A comparison of the sequences showed that the obtained 28S rDNA sequences have evolved at a much slower rate than the 16S rDNA, and that the former is better than the latter for resolving deep branching in the Odonata. Both molecular sources indicated that the Zygoptera are paraphyletic, and when a reasonable weighting for among-site rate variation was enforced for the 16S rDNA data set, E. superstes was placed between the two remaining major suborders, namely, Zygoptera and Anisoptera (dragonflies). Character reconstruction analysis suggests that multiple hits at the rapidly evolving sites in the 16S rDNA degenerated the phylogenetic signals of the data set. Key words: damselfly, dragonfly, molecular phylogeny. INTRODUCTION 2000; Artiss et al. -

Identification Guide to the Australian Odonata Australian the to Guide Identification
Identification Guide to theAustralian Odonata www.environment.nsw.gov.au Identification Guide to the Australian Odonata Department of Environment, Climate Change and Water NSW Identification Guide to the Australian Odonata Department of Environment, Climate Change and Water NSW National Library of Australia Cataloguing-in-Publication data Theischinger, G. (Gunther), 1940– Identification Guide to the Australian Odonata 1. Odonata – Australia. 2. Odonata – Australia – Identification. I. Endersby I. (Ian), 1941- . II. Department of Environment and Climate Change NSW © 2009 Department of Environment, Climate Change and Water NSW Front cover: Petalura gigantea, male (photo R. Tuft) Prepared by: Gunther Theischinger, Waters and Catchments Science, Department of Environment, Climate Change and Water NSW and Ian Endersby, 56 Looker Road, Montmorency, Victoria 3094 Published by: Department of Environment, Climate Change and Water NSW 59–61 Goulburn Street Sydney PO Box A290 Sydney South 1232 Phone: (02) 9995 5000 (switchboard) Phone: 131555 (information & publication requests) Fax: (02) 9995 5999 Email: [email protected] Website: www.environment.nsw.gov.au The Department of Environment, Climate Change and Water NSW is pleased to allow this material to be reproduced in whole or in part, provided the meaning is unchanged and its source, publisher and authorship are acknowledged. ISBN 978 1 74232 475 3 DECCW 2009/730 December 2009 Printed using environmentally sustainable paper. Contents About this guide iv 1 Introduction 1 2 Systematics -

© 2016 David Paul Moskowitz ALL RIGHTS RESERVED
© 2016 David Paul Moskowitz ALL RIGHTS RESERVED THE LIFE HISTORY, BEHAVIOR AND CONSERVATION OF THE TIGER SPIKETAIL DRAGONFLY (CORDULEGASTER ERRONEA HAGEN) IN NEW JERSEY By DAVID P. MOSKOWITZ A dissertation submitted to the Graduate School-New Brunswick Rutgers, The State University of New Jersey In partial fulfillment of the requirements For the degree of Doctor of Philosophy Graduate Program in Entomology Written under the direction of Dr. Michael L. May And approved by _____________________________________ _____________________________________ _____________________________________ _____________________________________ New Brunswick, New Jersey January, 2016 ABSTRACT OF THE DISSERTATION THE LIFE HISTORY, BEHAVIOR AND CONSERVATION OF THE TIGER SPIKETAIL DRAGONFLY (CORDULEGASTER ERRONEA HAGEN) IN NEW JERSEY by DAVID PAUL MOSKOWITZ Dissertation Director: Dr. Michael L. May This dissertation explores the life history and behavior of the Tiger Spiketail dragonfly (Cordulegaster erronea Hagen) and provides recommendations for the conservation of the species. Like most species in the genus Cordulegaster and the family Cordulegastridae, the Tiger Spiketail is geographically restricted, patchily distributed with its range, and a habitat specialist in habitats susceptible to disturbance. Most Cordulegastridae species are also of conservation concern and the Tiger Spiketail is no exception. However, many aspects of the life history of the Tiger Spiketail and many other Cordulegastridae are poorly understood, complicating conservation strategies. In this dissertation, I report the results of my research on the Tiger Spiketail in New Jersey. The research to investigate life history and behavior included: larval and exuvial sampling; radio- telemetry studies; marking-resighting studies; habitat analyses; observations of ovipositing females and patrolling males, and the presentation of models and insects to patrolling males. -

Cumulative Index of ARGIA and Bulletin of American Odonatology
Cumulative Index of ARGIA and Bulletin of American Odonatology Compiled by Jim Johnson PDF available at http://odonata.bogfoot.net/docs/Argia-BAO_Cumulative_Index.pdf Last updated: 14 February 2021 Below are titles from all issues of ARGIA and Bulletin of American Odonatology (BAO) published to date by the Dragonfly Society of the Americas. The purpose of this listing is to facilitate the searching of authors and title keywords across all issues in both journals, and to make browsing of the titles more convenient. PDFs of ARGIA and BAO can be downloaded from https://www.dragonflysocietyamericas.org/en/publications. The most recent three years of issues for both publications are only available to current members of the Dragonfly Society of the Americas. Contact Jim Johnson at [email protected] if you find any errors. ARGIA 1 (1–4), 1989 Welcome to the Dragonfly Society of America Cook, C. 1 Society's Name Revised Cook, C. 2 DSA Receives Grant from SIO Cook, C. 2 North and Central American Catalogue of Odonata—A Proposal Donnelly, T.W. 3 US Endangered Species—A Request for Information Donnelly, T.W. 4 Odonate Collecting in the Peruvian Amazon Dunkle, S.W. 5 Collecting in Costa Rica Dunkle, S.W. 6 Research in Progress Garrison, R.W. 8 Season Summary Project Cook, C. 9 Membership List 10 Survey of Ohio Odonata Planned Glotzhober, R.C. 11 Book Review: The Dragonflies of Europe Cook, C. 12 Book Review: Dragonflies of the Florida Peninsula, Bermuda and the Bahamas Cook, C. 12 Constitution of the Dragonfly Society of America 13 Exchanges and Notices 15 General Information About the Dragonfly Society of America (DSA) Cook, C. -

Microsoft Outlook
Joey Steil From: Leslie Jordan <[email protected]> Sent: Tuesday, September 25, 2018 1:13 PM To: Angela Ruberto Subject: Potential Environmental Beneficial Users of Surface Water in Your GSA Attachments: Paso Basin - County of San Luis Obispo Groundwater Sustainabilit_detail.xls; Field_Descriptions.xlsx; Freshwater_Species_Data_Sources.xls; FW_Paper_PLOSONE.pdf; FW_Paper_PLOSONE_S1.pdf; FW_Paper_PLOSONE_S2.pdf; FW_Paper_PLOSONE_S3.pdf; FW_Paper_PLOSONE_S4.pdf CALIFORNIA WATER | GROUNDWATER To: GSAs We write to provide a starting point for addressing environmental beneficial users of surface water, as required under the Sustainable Groundwater Management Act (SGMA). SGMA seeks to achieve sustainability, which is defined as the absence of several undesirable results, including “depletions of interconnected surface water that have significant and unreasonable adverse impacts on beneficial users of surface water” (Water Code §10721). The Nature Conservancy (TNC) is a science-based, nonprofit organization with a mission to conserve the lands and waters on which all life depends. Like humans, plants and animals often rely on groundwater for survival, which is why TNC helped develop, and is now helping to implement, SGMA. Earlier this year, we launched the Groundwater Resource Hub, which is an online resource intended to help make it easier and cheaper to address environmental requirements under SGMA. As a first step in addressing when depletions might have an adverse impact, The Nature Conservancy recommends identifying the beneficial users of surface water, which include environmental users. This is a critical step, as it is impossible to define “significant and unreasonable adverse impacts” without knowing what is being impacted. To make this easy, we are providing this letter and the accompanying documents as the best available science on the freshwater species within the boundary of your groundwater sustainability agency (GSA). -
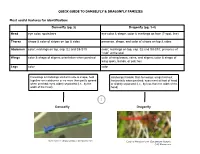
Dragonfly (Pg. 3-4) Head Eye Color
QUICK GUIDE TO DAMSELFLY & DRAGONFLY FAMILIES Most useful features for identification: Damselfly (pg. 2) Dragonfly (pg. 3-4) Head eye color; spots/bars eye color & shape; color & markings on face (T-spot, line) Thorax shape & color of stripes on top & sides presence, shape, and color of stripes on top & sides Abdomen color; markings on top, esp. S2 and S8-S10 color; markings on top, esp. S2 and S8-S10; presence of “club” at the end Wings color & shape of stigma; orientation when perched color of wing bases, veins, and stigma; color & shape of wing spots, bands, or patches Legs color color Forewings & hindwings similar in size & shape, held Hindwings broader than forewings; wings held out together over abdomen or no more than partly spread horizontally when perched; eyes meet at front of head when perched; eyes widely separated (i.e., by the or slightly separated (i.e., by less than the width of the width of the head) head) 1 Damselfly Dragonfly Vivid Dancer (Argia vivida); CAS Mazzacano Cardinal Meadowhawk (Sympetrum illotum); CAS Mazzacano DAMSELFLIES Wings narrow, stalked at base 2 Wings broad, colored, not stalked at base 3 Wings held askew Wings held together Broad-winged Damselfly when perched when perched (Calopterygidae); streams Spreadwing Pond Damsels (Coenagrionidae); (Lestidae); ponds ponds, streams Dancer (Argia); Wings held above abdomen; vivid colors streams 4 Wings held along Bluet (Enallagma); River Jewelwing (Calopteryx aequabilis); abdomen; mostly blue ponds CAS Mazzacano Dark abdomen with blue Forktail (Ischnura); tip; -

BIO-ECOLOGICAL STUDIES of MALAYSIAN ODONATES and an INTEGRATED TAXONOMIC STUDY on the GENUS Rhinocypha
BIO-ECOLOGICAL STUDIES OF MALAYSIAN ODONATES AND AN INTEGRATED TAXONOMIC STUDY ON THE GENUS Rhinocypha NOORHIDAYAH BINTI MAMATMalaya of FACULTY OF SCIENCE UNIVERSITY OF MALAYA UniversityKUALA LUMPUR 2018 BIO-ECOLOGICAL STUDIES OF MALAYSIAN ODONATES AND AN INTEGRATED TAXONOMIC STUDY ON THE GENUS Rhinocypha NOORHIDAYAH BINTI MAMAT Malaya THESIS SUBMITTED IN FULFILMENTof OF THE REQUIREMENT FOR THE DEGREE OF DOCTOR OF PHILOSOPHY INSTITUTE OF BIOLOGICAL SCIENCES FACULTY OF SCIENCE UNIVERSITY OF MALAYA UniversityKUALA LUMPUR 2018 UNIVERSITY OF MALAYA ORIGINAL LITERARY WORK DECLARATION Name of Candidate : NOORHIDAYAH BINTI MAMAT Matric No : SHC 140084 Name of Degree : DOCTOR OF PHILOSOPHY Title of Project Paper/Research Report/Dissertation/Thesis (“this Work”): BIO-ECOLOGICAL STUDIES OF MALAYSIAN ODONATES AND AN INTEGRATED TAXONOMIC STUDY ON THE GENUS Rhinocypha Field of Study: ECOLOGY & BIODIVERSITY (BIOLOGY & BIOCHEMISTRY) I do solemnly and sincerely declare that: (1) I am the sole author/writer of this Work; (2) This Work is original; (3) Any use of any work in which copyrightMalaya exists was done by way of fair dealing and for permitted purposes and any excerpt or extract from, or reference to or reproduction of any copyright work has been disclosed expressly and sufficiently and theof title of the Work and its authorship have been acknowledged in this Work; (4) I do not have any actual knowledge nor do I ought reasonably to know that the making of this work constitutes an infringement of any copyright work; (5) I hereby assign all and every rights in the copyright to this Work to the University of Malaya (“UM”), who henceforth shall be owner of the copyright in this Work and that any reproduction or use in any form or by any means whatsoever is prohibited without the written consent of UM having been first had and obtained; (6) I am fully aware that if in the course of making this Work I have infringed any copyright whether intentionally or otherwise, I may be subject to legal action or any other action as may be determined by UM. -

Estado Del Conocimiento De Los Odonata (Insecta) De Chile
BoletínH. NÚÑEZ del Museo y D. PINCHEIRA-DONOSO Nacional de Historia Natural, / Liolaemus Chile, confusus 56: 119-132, nueva (2007) especie de lagartija de Chile central 119 Estado DEL conocimiento DE LOS Odonata (Insecta) DE CHILE. ARIEL CAMOUSSEIGHT¹ y ALEJANDRO VERA² 1 Museo Nacional de Historia Natural, Casilla 787, Santiago. [email protected] 2 Universidad Metropolitana de Ciencias de la Educación, Casilla 147, Santiago. [email protected] RESUMEN Se analiza el estado actual del conocimiento de las especies del Orden Odonata registradas para Chile, destacando aspectos taxonómicos útiles para el trabajo limnológico, tales como el grado de discriminación que se puede lograr con los estados preimaginales y la distribución geográfica de las especies. Los resultados obtenidos revelan un total de 47 especies, adscritas a 23 géneros y 9 familias. De estas especies sólo han sido descritas 25 ninfas. El endemismo en Chile alcanzaría a un 29,8%. Palabras clave: Lista taxonómica, Odonata, Insectos acuáticos, Chile, Distribución. ABSTRACT Current state of knowledge of Odonata (Insecta) of Chile. The actual knowledge of the Order Odonata in Chile was studied, emphasizing in the taxonomic information useful for the limnological work such as the knowledge of the immature state and geographical distribution of species. A total of 47 species distributed in 23 genera and 9 families are recognized; the endemism reaches 29,8% of the species. Key words: Taxonomic list, Odonata, Aquatic insects, Chile, Distribution. INTRODUCCIÓN En el Simposio sobre el Estado del conocimiento de la biodiversidad acuática en Chile, organizado por la Sociedad Chilena de Limnología en el marco del XII Taller Nacional de Limnología celebrado el año 2003, se realizó una presentación preliminar del orden Odonata en Chile.