Geology, Geochemistry of Phosphatic Rocks Around Mardeora-Hirapur Area in Chhatarpur and Sagar Districts, Mp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

District Census Handbook, Chhatarpur, Parts X (A) & X
• CENSUS OF INDIA 1971 SERIES 10 MADHYA PRADESH DISTRICT CENSUS HANDBOOK PARTS X(A) & X(B) VILLAGE AND TOWN DIRECTORY VILLAGE AND TOWN-WISE PRIMARY CENSUS ABSTRACT CHHATARPUR DISTRICT A. K. PANDYA OF THE INDIAN ADMI]'.;)STRATIVE SERVICE DIRECTOR OF CENSUS OPERATIONS. MADHYA PRADESH PUBLISHED BY THE GOVERNMENT OF MADHYA PRADESH 1976 CONTENTS Pagt' 1. Preface i-ii 2. List of Abbreviations 1 3. Alphabetical List of Villages 3-15 ( j ) Laundi Tahsil 3-6 ( ii) Chhatarpur Tah,il 6-10 ( iii) Bijawar Tah~il 10··15 PART A 1. Explanatory Note 19-31 2. Village Directory (Amenities and Land-use) 32-83 ( i) Laundi Tahsil 32-45 ( ii) Chhatarpur Tahsil 46-63 ( iii) Bijawar Tahsil 64-83 3. Appendix to Village Directory 84-85 4. Town Directory 86-92 ( i) Status, Growth History and Functional Category of Towns 86 ( ii) Physical Aspects and Location of Towns 87 ( iii) Civic Finance 88 ( iv) Civic and other Amenities 89 ( v) Medical, Educational, Recreational and Cultural Facilities in Towns 90 ( vi) Trade, Commerce, Industry and B,l11king 91 (vii) Population by Religion and Scheduled Castes! Scheduled Tribes in Towns 92 5. Appendix to Town Directory 93 PART B 1. Explanatory Note 97·98 Z. Figures at a Glance 99 3. Primary Census Abstract 100-195 District Abstract 100-103 Laundi Tahsil 104·125 (Rural) 104-125 (Urban) Chhatarpur Tah~il 126-163 (Rural) 126-155 (Urban) 154-163 Bijawar Tahsil 164-195 (Rural) 164-193 (Urban) 194-195 1971 CENSUS PUBLICATIONS, MADHYA PRADESH (All the Census Publications of this Stat(· \\ill hear series No. -
College List.Pdf
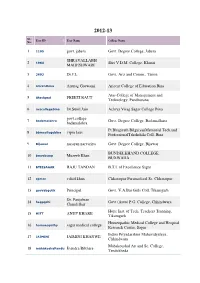
2012-13 Sr. User ID User Name College Name No. 1 1105 govt. jabera Govt. Degree College, Jabera SHRAVALLABH 2 1964 Shri V.D.M. College, Khurai MAHESHWARI 3 3603 Dr.J.L. Govt. Arts and Comm., Tamia 4 aricentbina Anurag Goswami Aricent College of Education Bina Atas College of Management and 5 Atashpnd PREETI RAUT Technology, Pandhurana 6 avscollegebina Dr.Sunil Jain Achrya Virag Sagar College Bina govt.collage 7 badamalehra Govt. Degree College, Badamalhara badamalehra Pt.Bhagirath BilgaiyanMemorial Tech.and 8 bbmcollegebina vipin laus ProfessionalTakshshila Coll, Bina 9 Bijawar narayan narvariya Govt. Degree College, Bijawar BUNDELKHAND COLLEGE, 10 bmvcbcmp Muzeeb Khan BUXWAHA 11 BTIESAGAR RAJU TANDAN B.T.I. of Excellence Sagar 12 cpmcc vahid khan Chhatarpur Paramedical Sc. Chhatarpur 13 govvabgctik Principal Govt. V.A.Bai Girls Coll. Tikamgarh Dr. Panjabrao 14 hegpgchi Govt (Auto) P.G. College, Chhindwara Chandelkar Hope Inst. of Tech. Teachers Tranning, 15 HITT ANUP KHARE Tikamgarh Homeopathic Medical College and Hospital 16 homoeopathy sagar medical college Research Center, Sagar Indira Priyadarshini Mahavidyalaya., 17 JAIMINI JAIMINI KHANWE Chhindwara Mahakoushal Art and Sc. College, 18 mahakoshaltendu Jitendra Bilthare Tendukheda Sr. User ID User Name College Name No. 19 MBSC471625 AJAY CHATURVEDI Maharaja Balvant Singh College, Rajnagar Maharaja Chhatarsal College of Education, 20 mcsm AJAY TIWARI Chhatarpur Veerangna Awanti Bai Law College, 21 naveen NAVEEN SOOD Chhatarpur ranjan karadbhajne Saupura College of Information and Bio- 22 parthnihal karadbhajne Technology, Sausar (Pipla) 23 pgclgharrai Aadh yadav Govt. Degree College, Harrai Pt. B.D. Memorial College of Education, 24 rakeshmishra rakesh mishra Makroniya 25 satpuralawcolle Jayendra Bharadwaj Satpura Law College, Chhindwara Sri Balaji College of Education, 26 shribalaji rajesh sahu Chhindwara 27 spn college Dr. -

Madhya Pradesh Size
Pradhan Mantri Awas Yojana (PMAY-U) 1 Meeting of CSMC Proposal for 2 Projects under Affordable Housing in Partnership (AHP) 164 Projects under Beneficiary Led Construction (BLC) 89 Housing For All Plan of Action (HFAPoA) 29th November, 2017 Urban Development & Housing Department Government of Madhya Pradesh 2 PMAY State Level Award 2 S. No. Award Category Awards 1 All Municipal Corporation 1st Prize - Rs. 1,00,000/- (Cities shall be evaluated for overall implementation of PMAY(U)) 2nd Prize - Rs. 50,000/- 3rd Prize - Rs. 25,000/- 2 Urban Local Bodies with Population above 50,000 1st Prize - Rs. 1,00,000/- (Cities shall be evaluated for overall implementation of PMAY(U)) 2nd Prize - Rs. 50,000/- 3rd Prize - Rs. 25,000/- 3 Rest of Urban Local Bodies 1st Prize - Rs. 1,00,000/- (Cities shall be evaluated for overall implementation of PMAY(U)) 2nd Prize - Rs. 50,000/- 3rd Prize - Rs. 25,000/- 4 Divisional Level 1st Prize - Rs. 1,00,000/- (Division shall be evaluated on the basis of overall performance of the cities of concerned division for implementation of PMAY(U)) 5 District Level 1st Prize - Rs. 1,00,000/- (District shall be evaluated on the basis of approval of beneficiary list, allotment of Patta2nd Prize - Rs. 50,000/- (As per Order No. - Hkwfe ghu@,Q-1-6/2017/18-3/18143 Dated - 10/07/2017) and3rd Prize - Rs. 25,000/- allotment of land projects approved under AHP, BLC and ISSR verticals of PMAY(U)) 6 Banks / HFIs 1st Prize - Rs. 1,00,000/- (Banks/HFIs shall be evaluation for Implementation of CLSS and lending Housing Loan to2nd Prize - Rs. -

Assessment of Domestic Pollution Load from Urban Agglomeration in Ganga Basin: Madhya Pradesh
Report Code: 063_GBP_IIT_EQP_S&R_13_VER 1_DEC 2014 Assessment of Domestic Pollution Load from Urban Agglomeration in Ganga Basin: Madhya Pradesh GRBMP: Ganga River Basin Management Plan by Indian Institutes of Technology IIT IIT IIT IIT IIT IIT IIT Bombay Delhi Guwahati Kanpur Kharagpur Madras Roorkee Report Code: 063_GBP_IIT_EQP_S&R_13_VER 1_DEC 2014 2 Report Code: 063_GBP_IIT_EQP_S&R_13_VER 1_DEC 2014 Preface In exercise of the powers conferred by sub-sections (1) and (3) of Section 3 of the Environment (Protection) Act, 1986 (29 of 1986), the Central Government has constituted National Ganga River Basin Authority (NGRBA) as a planning, financing, monitoring and coordinating authority for strengthening the collective efforts of the Central and State Government for effective abatement of pollution and conservation of the river Ganga. One of the important functions of the NGRBA is to prepare and implement a Ganga River Basin Management Plan (GRBMP). A Consortium of 7 Indian Institute of Technology (IIT) has been given the responsibility of preparing Ganga River Basin Management Plan (GRBMP) by the Ministry of Environment and Forests (MoEF), GOI, New Delhi. Memorandum of Agreement (MoA) has been signed between 7 IITs (Bombay, Delhi, Guwahati, Kanpur, Kharagpur, Madras and Roorkee) and MoEF for this purpose on July 6, 2010. This report is one of the many reports prepared by IITs to describe the strategy, information, methodology, analysis and suggestions and recommendations in developing Ganga River Basin Management Plan (GRBMP). The overall Frame Work for documentation of GRBMP and Indexing of Reports is presented on the inside cover page. There are two aspects to the development of GRBMP. -

Orchha State, Census Report, Vol-V
THE CENTRAL "INDIA STATE CENSUS SERIES Volume V ORCHHA STATE CENSUS REPORT FOR 1911 'TEXT AND TABLES COMPILED BY Majop C. E. LUARD, M.A. (Oxon), I. A., SUPERINTENDENT OF CENSUS OPERATIONS IN CENTRAL INDJX. ''l6ombal! ': PRINTED AT THE BRITISH INDI.A PRESS, MAZAGAON. 1.913 PREFACE. The Census, with which this R'2port deals, is the fourth regular enumeration of the population of the Orchha Sbte, the first being in 1881. The dates on whic h the four Censuses were taken :.;re noted below:- 17th February 188!. 1st March 1901. 26th February Ib91. 10fh March 1911. All the [mo' have been ~yn(hronous with the Censuses taken in British India. In the first two CemUSl!S a simple form of the Schedule consisting of 8 columns was adopted requiring information on (1) ~umber, (2) Name, (3) Religion, (4) Sex, (5) Age, (6) Caste, Tribe, or H;:we, (7) Birth-place and (8) Occupation. In 1901 as well as on the present occasion tl-;P British India Schedule was exactly applied and the results were incorporated in Cr ntl'al j ndia Imperial Tables. As on previous occasions the first ptep taken, after it was notified that a Census was to be taken, was the prepnration of a list of all villages in the State in the prescribed form for the formation of Ccn;;us Division8. This was done early in March 1910, after which the DarLa1' a:rpointed, as its Census Officer, Lala Ujagar Chand, who had previous experience of this work. Lala Ujagar Chand with two Supervisors attended the training class opened at Indore for the instruction of C('nsus Officers. -

Ken- Betwa Link Project, Phase-I
VOLUME - III KEN- RESETTLEMENT AND REHABILITATON: PROJECT AFFECTED FAMILIES BETWA ECONOMIC REHABILITATION PLAN (PAFERP) LINK PROJECT, PHASE-I Submitted to NATIONAL WATER DEVELOPMENT AGENCY (NWDA) Prepared by RESETTLEMENT AND REHABILITATON PLAN AGRICULTURAL FINANCE CORPORATION LTD, (R&R) MUMBAI, INDIA December, 2014 INDIA NATIONAL WATER DEVELOPMENT AGENCY (A Govt. of India society under Ministry of Water Resources) COMPREHENSIVE ENVIRONMENT IMPACT ASSESSMENT STUDY OF PROPOSED KEN-BETWA LINK PROJECT PHASE-I VOLUME – III RESETTLEMENT AND REHABILITATON: PROJECT AFFECTED FAMILIES ECONOMIC REHABILITATION PLAN (PAFERP) as per “The Right to Fair Compensation and Transparency in Land Acquisition, Rehabilitation and Resettlement Act 2013”. CONTENTS Chapter No. Title Page(s) I Introduction 1-3 II Socio – Economic Status in Command Area 4-18 III Socio-Economic Environment in Submergence Area 19-27 IV Resettlement and Economic Rehabilitation Plan 28-39 V Training 40-43 VI Organization and Management 44-49 VII Monitoring and Evaluation 50-53 VIII Implementation Schedule 54-55 IX Project Cost 56-57 Annexures Annexure Title Page No. No. II.1 Existing Cropping Pattern in the Command Area 58 II.2 Existing Crop Production in the Command Area 59 II.3 Agriculture Input Pattern and Economics of Cultivation in the 60-63 Command Area III.1 Demography Population of Project Affected Families 64 III.3 Caste-wise distribution of Households and its Population in the 65 Project Submergence Areas IV.1 Details of Land Acquisition of Daudhan dam, Link Canal, LBC 66-67 system and power houses CHAPTER - I INTRODUCTION Background 1.01 While the projects are the building blocks for development, the irrigation reservoir projects at times result in submersion of houses and house sites rendering people homeless besides causing loss of valuable agricultural land affecting the over all social fabric of the affected people. -

Service Electors Voter List
FINAL ELECTORAL ROLL - 2021 STATE - (S12) MADHYA PRADESH No., Name and Reservation Status of Assembly Constituency: 53-MALHARA(GEN) Last Part No., Name and Reservation Status of Parliamentary Service Constituency in which the Assembly Constituency is located: 7-DAMOH(GEN) Electors 1. DETAILS OF REVISION Year of Revision : 2021 Type of Revision : Special Summary Revision Qualifying Date :01/01/2021 Date of Final Publication: 15/01/2021 2. SUMMARY OF SERVICE ELECTORS A) NUMBER OF ELECTORS 1. Classified by Type of Service Name of Service No. of Electors Members Wives Total A) Defence Services 82 1 83 B) Armed Police Force 0 0 0 C) Foreign Service 0 0 0 Total in Part (A+B+C) 82 1 83 2. Classified by Type of Roll Roll Type Roll Identification No. of Electors Members Wives Total I Original Mother roll Integrated Basic roll of revision 82 1 83 2021 II Additions Supplement 1 After Draft publication, 2021 0 0 0 List Sub Total: 0 0 0 III Deletions Supplement 1 After Draft publication, 2021 0 0 0 List Sub Total: 0 0 0 Net Electors in the Roll after (I + II - III) 82 1 83 B) NUMBER OF CORRECTIONS/MODIFICATION Roll Type Roll Identification No. of Electors Supplement 1 After Draft publication, 2021 0 Total: 0 Elector Type: M = Member, W = Wife Page 1 Final Electoral Roll, 2021 of Assembly Constituency 53-MALHARA (GEN), (S12) MADHYA PRADESH A . Defence Services Sl.No Name of Elector Elector Rank Husband's Address of Record House Address Type Sl.No. Officer/Commanding Officer for despatch of Ballot Paper (1) (2) (3) (4) (5) (6) (7) Assam Rifles 1 BHAGWAN -

Mesoproterozoic Diamondiferous Ultramafic Pipes at Majhgawan And
Mesoproterozoic diamondiferous ultramafic pipes at Majhgawan and Hinota, Panna area, central India: Key to the nature of sub-continental lithospheric mantle beneath the Vindhyan basin N V Chalapathi Rao EPMA Laboratory, Mineralogy Section, Ore Dressing Division, Indian Bureau of Mines, Nagpur 440 016, India. e-mail: [email protected] Amongst all the perceptible igneous manifestations (volcanic tuffs and agglomerates, minor rhy- olitic flows and andesites, dolerite dykes and sills near the basin margins, etc.) in the Vindhyan basin, the two Mesoproterozoic diamondiferous ultramafic pipes intruding the Kaimur Group of sediments at Majhgawan and Hinota in the Panna area are not only the most conspicuous but also well-known and have relatively deeper mantle origin. Hence, these pipes constitute the only yet available ‘direct’ mantle samples from this region and their petrology, geochemistry and iso- tope systematics are of profound significance in understanding the nature of the sub-continental lithospheric mantle beneath the Vindhyan basin. Their emplacement age (∼1100 Ma) also con- stitutes the only reliable minimum age constrain on the Lower Vindhyan Group of rocks. The Majhgawan and Hinota pipes share the petrological, geochemical and isotope characteristics of kimberlite, orangeite (Group II kimberlite) and lamproite and hence are recognised as belonging to a ‘transitional kimberlite–orangeite–lamproite’ rock type. The name majhagwanite has been proposed by this author to distinguish them from other primary diamond source rocks. The par- ent magma of the Majhgawan and Hinota pipes is envisaged to have been derived by very small (<1%) degrees of partial melting of a phlogopite-garnet lherzolite source (rich in titanium and barium) that has been previously subjected to an episode of initial depletion (extensive melting during continent formation) and subsequent metasomatism (enrichment). -

Madhya Pradesh.Xlsx
Madhya Pradesh S.No. District Name of the Address Major Activity Broad NIC Owner Emplo Code Establishment Description Activity ship yment Code Code Class Interval 130MPPGCL (POWER SARNI DISTT POWER 07 351 4 >=500 HOUSE) BETUL(M.P.) DISTT GENERATION PLANT BETUL (M.P.) 460447 222FORCE MOTORS ARCADY, PUNE VEHICAL 10 453 2 >=500 LTD. MAHARASHTRA PRODUCTION 340MOIL BALAGHAT OFFICER COLONEY MAINING WORK 05 089 4 >=500 481102 423MARAL YARN KHALBUJURG A.B. CLOTH 06 131 2 >=500 FACTORY ROAD MANUFACTRING 522SHRI AOVRBINDO BHOURASALA HOSPITAL 21 861 3 >=500 MEDICAL HOSPITAL SANWER ROAD 453551 630Tawa mines pathakheda sarni COOL MINING WORK 05 051 1 >=500 DISTT BETUL (M.P.) 460447 725BHARAT MATA HIGH BAJRANG THREAD 06 131 1 >=500 SCHOOL MANDAWAR MOHHALLA 465685 PRODUCTION WORK 822S.T.I INDIA LTD. PITHAMPUR RING MAKING OF 06 141 2 >=500 ROAD 453332 READYMADE CLOTHS 921rosi blue india pvt.ltd sector no.1 454775 DAYMAND 06 239 3 >=500 COTIND&POLISING 10 30 SHOBHAPUR MINSE PATHAKERA DISTT COL MININING 05 051 4 >=500 BETUL (M.P.) 440001 11 38 LAND COLMINCE LINE 0 480442 KOLMINCE LAND 05 089 1 >=500 OFFICE,MOARI INK SCAPE WORK 12 44 OFFICE COAL MINES Bijuri OFFICE COAL COAL MINES 05 051 1 >=500 SECL BILASPUR MINES SECL BILASPUR Korja Coliery Bijuri 484440 13 38 W.C.L. Dist. Chhindwara COL MINING 05 051 4 >=500 480559 14 22 SHIWALIK BETRIES PANCHDERIYA TARCH FACTORY 06 259 2 >=500 PVT. LTD. 453551 15 33 S.S.E.C.N. WEST Katni S.S.E.C.N. RIPERING OF 10 454 1 >=500 RAILWAY KATNI WEST RAILWAY MALGADI DEEBBE KATNI Nill 483501 16 44 Jhiriya U.G.Koyla Dumarkachar Jhiriya CAOL SUPPLY WORK 06 239 4 >=500 khadan U.G.Koyla khadan Dumarkachar 484446 17 23 CENTURY YARN SATRATI 451228 CENTURY YARN 06 141 4 >=500 18 21 ret spean pithampur 454775 DHAGA PRODUCTS 06 131 4 >=500 19 21 hdfe FEBRICATION PITHAMPUR 454775 FEBRICATION 06 141 2 >=500 20 29 INSUTATOR ILE. -
Sr No. Branch Code Name of the Branch Complete Postal Address
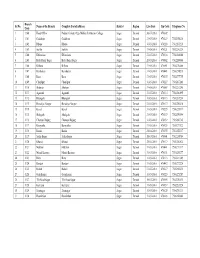
Branch Sr No. Name of the Branch Complete Postal Address District Region Live Date Pin Code Telephone No. Code 1 1100 Head Office Poddar Colony, Opp. Mahila Polytecnic College Sagar Damoh 30-07-2010 470002 2 1101 Gadakota Gadakota Sagar Damoh 31-05-2010 470229 7585258238 3 1102 Dhana Dhana Sagar Damoh 31-05-2010 470228 7582285218 4 1103 Surkhi Surkhi Sagar Damoh 31-05-2010 470221 7582280224 5 1104 Khimalasa Khimalasa Sagar Damoh 31-05-2010 470118 7581284348 6 1105 Bada Bazar Sagar Bada Bazar Sagar Sagar Damoh 20-03-2010 470002 7582249046 7 1106 Bilhara Bilhara Sagar Damoh 31-05-2010 470051 7584270408 8 1107 Barokalan Barokalan Sagar Damoh 31-05-2010 470441 7581274213 9 1108 Bara Bara Sagar Damoh 31-05-2010 470335 7583277755 10 1109 Chandpur Chandpur Sagar Damoh 31-05-2010 470227 7585287241 11 1110 Shahpur Shahpur Sagar Damoh 31-05-2010 470669 7582282288 12 1111 Agasaud Agasaud Sagar Damoh 31-05-2010 470114 7580280895 13 1112 Bhangarh Bhangarh Sagar Damoh 31-05-2010 470113 7580283234 14 1113 Barodiya Nongar Barodiya Nongar Sagar Damoh 31-05-2010 470117 7581290410 15 1114 Kesali Kesali Sagar Damoh 31-05-2010 470235 7586224314 16 1115 Shahgarh Shahgarh Sagar Damoh 31-05-2010 470339 7583259194 17 1116 Chanaua Bujurg Chanaua Bujurg Sagar Damoh 31-05-2010 470232 7585290725 18 1117 Barayatha Barayatha Sagar Damoh 31-05-2010 470335 7583277122 19 1118 Banda Banda Sagar Damoh 28-04-2010 470335 7583252237 20 1119 Sadar Sagar Sadar Sagar Sagar Damoh 18-03-2010 470001 7582238764 21 1120 Khurai Khurai Sagar Damoh 28-04-2010 470117 7581240824 22 1121 -

Madhya Pradesh Urban Services Improvement Project: Package 6D
Initial Environmental Examination Document Stage: Draft Project Number: 42486-016 June 2016 IND: Madhya Pradesh Urban Services Improvement Program – Water Supply Improvement in Buxwaha, Hatta and Pawai Package No: MPUSIP-6D Prepared by Madhya Pradesh Urban Development Company, Government of Madhya Pradesh for the Asian Development Bank. This initial environmental examination is a document of the borrower. The views expressed herein do not necessarily represent those of ADB's Board of Directors, Management, or staff, and may be preliminary in nature. In preparing any country program or strategy, financing any project, or by making any designation of or reference to a particular territory or geographic area in this document, the Asian Development Bank does not intend to make any judgments as to the legal or other status of any territory or area. Draft Initial Environmental Examination June 2016 IND: Madhya Pradesh Urban Services Improvement Program –Subproject of Water Supply Improvement in Buxwaha, Hatta and Pawai (Package 6D) Prepared by Project Management Unit, Madhya Pradesh Urban Development Company, Government of Madhya Pradesh for the Asian Development Bank CURRENCY EQUIVALENTS (as of 1 Dec2015) Currency unit – Conversion INR1.00 = $.0.015 $1.00 = INR 66.00 Abbreviations AC – Asbestos Cement ADB – Asian Development Bank ASO – Assistant Safeguards Officer CFE – Consent for Establishment CFO – Consent for Operation CPCB Central Pollution Control Board EA – Executing Agency EAC – Expert Appraisal Committee EC – Environmental Clearance EHS – Environmental Health & Safety EIA – Environmental Impact Assessment EMP – Environmental Management Plan; GOI – Government of India GoMP – Government of Madhya Pradesh IA – Implementing Agency IEE – Initial Environmental Examination; NP – Nagar Parishad LPCD – Liters per Capita per Day MLD – Million Liters per Day MOEF – Ministry of Environment and Forest MPPCB – Madhya Pradesh Pollution Control Board MPUDC – Madhya Pradesh Urban Development Company Ltd. -

Tikamgarh.Pdf
Developmental Works - Some Glimpses INDIAN RAILWAYS NEW RAILWAYS Inauguration of new train 22164-Mahamana Express A progressive journey since 2014 MADHYA PRADESH ON FAST TRACK DEVELOPMENT : 2014-PRESENT INPUTS BY RAILWAY IN TIKAMGARH PARLIAMENTARY CONSTITUENCY INFRASTRUCTURE ENHANCEMENT : TIKAMGARH PARLIAMENTARY CONSTITUENCY v New line Projects : New line projects of 726 Kms for `11,903 crore A. ASSEMBLY SEGMENTS sanctioned since 2014 Tikamgarh, Jatara, Prithvipur, Niwari, Kahargapur, Maharajpur, v Doubling Projects : Doubling projects of 1,722 Kms for `18,424 crore Chhatarpur, Bijawar sanctioned since 2014 v Electrication Projects : Electrication projects of 3,111 Kms for `3,118 B. WORKS COMPLETED SINCE 2014 B1. Infrastructural Development crore sanctioned since 2014 v New line between Lalitpur to Khajuraho (114 Kms). Total cost of work for v Rail Fly Over Projects : Rail yover projects of 68 Kms for `1,801 crore 160 KM is 850 crore. sanctioned since 2014 B2. Improvements In Passengers Amenities MADHYA PRADESH CONNECT : PASSENGER FOCUS v 4 nos stainless steel bench provided at Niwari station. v v New Trains : 107 new trains introduced since 2014 Provision of facilities for handicapped passenger at Tikamgarh v Trains Extended : 53 trains extended since 2014 v Frequency Increased : for 13 trains since 2014 B3. Improvements In Train Services Introduction of New Trains SAFETY : PRIME CONCERN Train no. 19663/19664 khajuraho indore express started w.e.f. 17.02.19 v Use of drone by WCR in Madhya Pradesh is rst in India for disaster Stoppage to passenger trains v Train no. 22164-Mahamana Express at Tikamgarh. management, crowd management and project monitoring etc. v Train no.19663/64 KURJ-INDB Express at Tikamgarh & MCSC v Use of GPS Tracker by WCR in Madhya Pradesh is rst for India.