Geochemistry of CO2-Rich Gases Venting from Submarine Volcanism
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SPLOS/240 Meeting of States Parties
United Nations Convention on the Law of the Sea SPLOS/240 Meeting of States Parties Distr.: General 12 March 2012 Original: English Twenty-second Meeting New York, 4-11 June 2012 Curricula vitae of candidates nominated by States Parties for election to the Commission on the Limits of the Continental Shelf Note by the Secretary-General 1. The Secretary-General has the honour to submit the curricula vitae of the candidates nominated by States Parties for the election of 21 members of the Commission on the Limits of the Continental Shelf for a five-year term beginning on 16 June 2012 (see annex). The names and nationalities of the candidates are as follows: Mohammad bin Hamid Al-Harbi (Saudi Arabia) Muhammad Arshad (Pakistan) Mario Juan A. Aurelio (Philippines) Lawrence Folajimi Awosika (Nigeria) Galo Carrera (Mexico) Francis L. Charles (Trinidad and Tobago) Ivan F. Glumov (Russian Federation) Richard Thomas Haworth (Canada and United Kingdom of Great Britain and Northern Ireland) Martin Vang Heinesen (Denmark) Emmanuel Kalngui (Cameroon) Lu Wenzheng (China) Mazlan Bin Madon (Malaysia) Estevao Stefane Mahanjane (Mozambique) Jair Alberto Ribas Marques (Brazil) Simon Njuguna (Kenya) Isaac Owusu Oduro (Ghana) Yong Ahn Park (Republic of Korea) Carlos Marcelo Paterlini (Argentina) Sivaramakrishnan Rajan (India) Walter R. Roest (Netherlands) Luis Somoza Losada (Spain) Nguyen Nhu Trung (Viet Nam) Tetsuro Urabe (Japan) 2. Information concerning the nominations and the election is contained in documents SPLOS/238 and SPLOS/239 and Add.1. 12-26137 (E) 140512 *1226137* SPLOS/240 Annex Curricula vitae of candidates* Mohammad bin Hamid Al-Harbi (Saudi Arabia) Position: Director General of Marine Surveying at Saudi Arabia’s General Directorate for Marine Geodesy Education: 1. -

Microbiology of Seamounts Is Still in Its Infancy
or collective redistirbution of any portion of this article by photocopy machine, reposting, or other means is permitted only with the approval of The approval portionthe ofwith any articlepermitted only photocopy by is of machine, reposting, this means or collective or other redistirbution This article has This been published in MOUNTAINS IN THE SEA Oceanography MICROBIOLOGY journal of The 23, Number 1, a quarterly , Volume OF SEAMOUNTS Common Patterns Observed in Community Structure O ceanography ceanography S BY DAVID EmERSON AND CRAIG L. MOYER ociety. © 2010 by The 2010 by O ceanography ceanography O ceanography ceanography ABSTRACT. Much interest has been generated by the discoveries of biodiversity InTRODUCTION S ociety. ociety. associated with seamounts. The volcanically active portion of these undersea Microbial life is remarkable for its resil- A mountains hosts a remarkably diverse range of unusual microbial habitats, from ience to extremes of temperature, pH, article for use and research. this copy in teaching to granted ll rights reserved. is Permission S ociety. ociety. black smokers rich in sulfur to cooler, diffuse, iron-rich hydrothermal vents. As and pressure, as well its ability to persist S such, seamounts potentially represent hotspots of microbial diversity, yet our and thrive using an amazing number or Th e [email protected] to: correspondence all end understanding of the microbiology of seamounts is still in its infancy. Here, we of organic or inorganic food sources. discuss recent work on the detection of seamount microbial communities and the Nowhere are these traits more evident observation that specific community groups may be indicative of specific geochemical than in the deep ocean. -

Geochemistry of Hydrothermal Fluids from the PACMANUS, Northeast Pual and Vienna Woods Hydrothermal Fields, Manus Basin, Papua New Guinea Eoghan P
Bridgewater State University Virtual Commons - Bridgewater State University Geological Sciences Faculty Publications Geological Sciences Department 2011 Geochemistry of hydrothermal fluids from the PACMANUS, Northeast Pual and Vienna Woods hydrothermal fields, Manus Basin, Papua New Guinea Eoghan P. Reeves Jeffrey S. Seewald Peter Saccocia Bridgewater State University, [email protected] Wolfgang Bach Paul R. Craddock See next page for additional authors Follow this and additional works at: http://vc.bridgew.edu/geology_fac Part of the Earth Sciences Commons Virtual Commons Citation Reeves, Eoghan P.; Seewald, Jeffrey S.; Saccocia, Peter; Bach, Wolfgang; Craddock, Paul R.; Shanks, Wayne C. III; Sylva, Sean P.; Walsh, Emily; Pichler, Thomas; and Rosner, Martin (2011). Geochemistry of hydrothermal fluids from the PACMANUS, Northeast Pual and Vienna Woods hydrothermal fields, Manus Basin, Papua New Guinea. In Geological Sciences Faculty Publications. Paper 9. Available at: http://vc.bridgew.edu/geology_fac/9 This item is available as part of Virtual Commons, the open-access institutional repository of Bridgewater State University, Bridgewater, Massachusetts. Authors Eoghan P. Reeves, Jeffrey S. Seewald, Peter Saccocia, Wolfgang Bach, Paul R. Craddock, Wayne C. Shanks III, Sean P. Sylva, Emily Walsh, Thomas Pichler, and Martin Rosner This article is available at Virtual Commons - Bridgewater State University: http://vc.bridgew.edu/geology_fac/9 Available online at www.sciencedirect.com Geochimica et Cosmochimica Acta 75 (2011) 1088–1123 www.elsevier.com/locate/gca Geochemistry of hydrothermal fluids from the PACMANUS, Northeast Pual and Vienna Woods hydrothermal fields, Manus Basin, Papua New Guinea Eoghan P. Reeves a,b,⇑, Jeffrey S. Seewald b, Peter Saccocia c, Wolfgang Bach d, Paul R. -

USGS Open-File Report 2009-1133, V. 1.2, Table 3
Table 3. (following pages). Spreadsheet of volcanoes of the world with eruption type assignments for each volcano. [Columns are as follows: A, Catalog of Active Volcanoes of the World (CAVW) volcano identification number; E, volcano name; F, country in which the volcano resides; H, volcano latitude; I, position north or south of the equator (N, north, S, south); K, volcano longitude; L, position east or west of the Greenwich Meridian (E, east, W, west); M, volcano elevation in meters above mean sea level; N, volcano type as defined in the Smithsonian database (Siebert and Simkin, 2002-9); P, eruption type for eruption source parameter assignment, as described in this document. An Excel spreadsheet of this table accompanies this document.] Volcanoes of the World with ESP, v 1.2.xls AE FHIKLMNP 1 NUMBER NAME LOCATION LATITUDE NS LONGITUDE EW ELEV TYPE ERUPTION TYPE 2 0100-01- West Eifel Volc Field Germany 50.17 N 6.85 E 600 Maars S0 3 0100-02- Chaîne des Puys France 45.775 N 2.97 E 1464 Cinder cones M0 4 0100-03- Olot Volc Field Spain 42.17 N 2.53 E 893 Pyroclastic cones M0 5 0100-04- Calatrava Volc Field Spain 38.87 N 4.02 W 1117 Pyroclastic cones M0 6 0101-001 Larderello Italy 43.25 N 10.87 E 500 Explosion craters S0 7 0101-003 Vulsini Italy 42.60 N 11.93 E 800 Caldera S0 8 0101-004 Alban Hills Italy 41.73 N 12.70 E 949 Caldera S0 9 0101-01= Campi Flegrei Italy 40.827 N 14.139 E 458 Caldera S0 10 0101-02= Vesuvius Italy 40.821 N 14.426 E 1281 Somma volcano S2 11 0101-03= Ischia Italy 40.73 N 13.897 E 789 Complex volcano S0 12 0101-041 -

Microbial Colonization of Basaltic Glasses in Hydrothermal Organic-Rich Sediments at Guaymas Basin
Microbial colonization of basaltic glasses in hydrothermal organic-rich sediments at Guaymas Basin. Nolwenn Callac, C´elineRommevaux-Jestin, Olivier Rouxel, Fran¸coise Lesongeur, C´elineLiorzou, Claire Bollinger, Antony Ferrant, Anne Godfroy To cite this version: Nolwenn Callac, C´elineRommevaux-Jestin, Olivier Rouxel, Fran¸coiseLesongeur, C´elineLior- zou, et al.. Microbial colonization of basaltic glasses in hydrothermal organic-rich sediments at Guaymas Basin.. Frontiers in microbiology, Frontiers Research Foundation, 2013, 4, pp.250. <10.3389/fmicb.2013.00250>. <insu-00933493> HAL Id: insu-00933493 https://hal-insu.archives-ouvertes.fr/insu-00933493 Submitted on 20 Jan 2014 HAL is a multi-disciplinary open access L'archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destin´eeau d´ep^otet `ala diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publi´esou non, lished or not. The documents may come from ´emanant des ´etablissements d'enseignement et de teaching and research institutions in France or recherche fran¸caisou ´etrangers,des laboratoires abroad, or from public or private research centers. publics ou priv´es. ORIGINAL RESEARCH ARTICLE published: 27 August 2013 doi: 10.3389/fmicb.2013.00250 Microbial colonization of basaltic glasses in hydrothermal organic-rich sediments at Guaymas Basin Nolwenn Callac 1,2,3,4, Céline Rommevaux-Jestin 5, Olivier Rouxel 4,6, Françoise Lesongeur 1,2,3, Céline Liorzou 4, Claire Bollinger 7, Antony Ferrant -

Hydrothermal Activity in the Okinawa Trough Back-Arc Basin: Geological Background 27 and Hydrothermal Mineralization
Hydrothermal Activity in the Okinawa Trough Back-Arc Basin: Geological Background 27 and Hydrothermal Mineralization Jun-ichiro Ishibashi, Fumihiko Ikegami, Takeshi Tsuji, and Tetsuro Urabe Abstract The Okinawa Trough is a back-arc basin behind the Ryukyu trench-arc system and located along the eastern margin of the Eurasian continent. Sulfide and sulfate mineralization associated with hydrothermal activity has been recognized in ten hydrothermal fields in the Okinawa Trough. Hydrothermal mineralization recognized in these fields is commonly represented by coexisting occurrence of zinc- and lead-enriched polymetallic sulfides and abundant sulfate minerals. The mineralogy and geochemical signatures present has led researchers to suggest these areas may be a modern analogue for the formation of ancient Kuroko-type volcanogenic massive sulfide (VMS) deposits. Recent seafloor drilling during IODP (Integrated Ocean Drilling Program) Expedition 331 documented the subseafloor structure of a hydrothermal system at the Iheya North Knoll. Mineral textures and hydro- thermal assemblages present in the drilled cores obtained from a hydrothermal mound in the proximal area are consistent with Kuroko-type mineralization. Based on geochemical studies, the intra-field diversity of mineralization commonly recognized in the Okinawa Trough can be explained by subseafloor phase separation of the hydrothermal fluid, which reflects shallow water depth (from 700 to 1,600 m). The subseafloor phase separation may. play an important role to accumulate metal elements beneath the seafloor. Based on geophysical and geological studies, the Okinawa Trough is considered a back-arc basin in the rifting stage. Such a tectonic setting is characterized by development of normal faulting in brittle continental crust and frequent intrusion of a magma, which can be expected to provide favorable environment for development of a hydrothermal system Keywords Back-arc rifting Kuroko-type deposit Mineralization Phase separation Volcanic massive sulfide J.-i. -

Volcanic and Hydrothermal Processes in Submarine Calderas: the Kulo Lasi Example (SW Pacific)
1 Ore Geology Reviews Archimer August 2018, Volume 99, Pages 314-343 http://dx.doi.org/10.1016/j.oregeorev.2018.06.006 http://archimer.ifremer.fr http://archimer.ifremer.fr/doc/00445/55633/ © 2018 Elsevier B.V. All rights reserved. Volcanic and hydrothermal processes in submarine calderas: the Kulo Lasi example (SW Pacific) Fouquet Yves 1, *, Pelleter Ewan 1, Konn Cecile 1, Chazot Gilles 2, Dupré Stephanie 1, Alix Anne-Sophie 1, Chéron Sandrine 1, Donval Jean-Pierre 1, Guyader Vivien 1, Etoubleau Joel 1, Charlou Jean-Luc 1, Labanieh Shasa 2, Scalabrin Carla 1 1 Ifremer, REM-UGM-LGM - ZI de la pointe du diable, CS 10070, 29280 Plouzané, France 2 Université de Brest - IUEM, ZI de la pointe du diable, 29280 Plouzané, France * Corresponding author : Yves Fouquet, email address : [email protected] Abstract : The study area is located at the transition between the northern end of the Tonga Trench and the North Fiji fracture zone, where tectonic movements are reputed to be the fastest in the world. To the southeast of Futuna Island, a broad area of volcanism occurs within a region characterized by a change in the tectonic fabric between a NE-SW oriented volcanic graben and the N-S oriented Alofi ridge. In 2010, the active volcano Kulo Lasi, which represents the most recent volcanic episode in the Futuna area, was discovered in the center of this extensive volcanic zone. Kulo Lasi is a 20 km diameter shield volcano that rises 400 m above the seafloor. It is composed of basaltic to trachy-andesitic lava with no obvious geochemical affinity with the Tonga subduction that occurs 500 km to the east. -
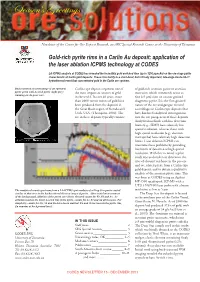
Ore Solutions 15
Season’s Greetings Newsletter of the Centre for Ore Deposit Research, an ARC Special Research Centre at the University of Tasmania Gold-rich pyrite rims in a Carlin Au deposit: application of the laser ablation ICPMS technology at CODES LA-ICPMS analysis at CODES has revealed the incredibly gold-enriched rims (up to 1200 ppm Au) on the ore-stage pyrite characteristic of Carlin gold deposits. These rims testify to a short-lived, but critically important, late-stage Au-As-Sb-Tl hydrothermal event that concentrated gold in the Carlin ore systems. Backscattered electron image of an euhedral Carlin-type deposits represent one of of gold-rich arsenian pyrite or arsenian pyrite grain with As-rich pyrite (light grey) the most important sources of gold marcasite, which commonly occur as rimming an As-poor core. in the world. In over 40 years, more fine (<5 μm) rims on coarser grained than 2000 metric tonnes of gold have diagenetic pyrite. It is the fine-grained been produced from the deposits in nature of the ore and gangue mineral the Great Basin region of Nevada and assemblages in Carlin-type deposits that Utah, USA. (Thompson, 2002). The have hindered analytical investigations ore in these deposits typically consists into the ore paragenesis of these deposits. Analytical methods with low detection limits (e.g., SIMS) have relatively low spatial resolution, whereas those with high spatial resolution (e.g., electron microprobe) have relatively high detection limits. Laser ablation ICPMS can overcome these problems by providing low limits of detection at high spatial resolution. With this in mind, a pilot study was undertaken to determine the sites of element residence in the pre-ore and ore related pyrite from a Carlin-type gold deposit, and to obtain a qualitative analysis of the arsenian pyrite rims. -

Brothers Volcano, Southern Kermadec Arc, New Zealand
1097-1133 3/31/06 10:38 AM Page 1097 ©2005 Society of Economic Geologists, Inc. Economic Geology, v. 100, pp. 1097–1133 Evolution of a Submarine Magmatic-Hydrothermal System: Brothers Volcano, Southern Kermadec Arc, New Zealand C. E. J. DE RONDE,† Institute of Geological and Nuclear Sciences, P.O. Box 31-312, Lower Hutt, New Zealand M. D. HANNINGTON, Department of Earth Sciences, University of Ottawa, Ottawa, Canada K1N 6N5 P. S TOFFERS, Institute of Geosciences, Christian-Albrechts University of Kiel, Olshausenstrasse 40, 24118 Kiel, Germany I. C. WRIGHT, National Institute of Water and Atmospheric Research, P.O. Box 14-901, Wellington, New Zealand R. G. DITCHBURN, A. G. REYES, Institute of Geological and Nuclear Sciences, P.O. Box 31-312, Lower Hutt, New Zealand E. T. BAKER, Pacific Marine Environmental Laboratory, National Oceanic and Atmospheric Administration, 7600 Sand Point Way, NE Bldg. 3, Seattle, Washington 98115-6349 G. J. MASSOTH, Institute of Geological and Nuclear Sciences, P.O. Box 31-312, Lower Hutt, New Zealand J. E. LUPTON, Pacific Marine Environmental Laboratory, National Oceanic and Atmospheric Administration, 2115 S.E. OSU Drive, Newport, OR 97365-5258 S. L. WALKER, Pacific Marine Environmental Laboratory, National Oceanic and Atmospheric Administration, 7600 Sand Point Way NE, Bldg. 3, Seattle, Washington 98115-6349 R. R. GREENE, Pacific Marine Environmental Laboratory, National Oceanic and Atmospheric Administration, 2115 S.E. OSU Drive, Newport, OR 97365-5258 C. W. R. SOONG, Institute of Geological and Nuclear Sciences, P.O. Box 31-312, Lower Hutt, New Zealand J. ISHIBASHI, Faculty of Science, Kyushu University, Japan G. -

Polymetallic Massive Sulphide Deposits at the Modern Seafloor
Polymetallic Massive Sulphides and Cobalt-Rich Ferromanganese Crusts: Status and Prospects ISA Technical Study: No.2 International Seabed Authority Polymetallic Massive Sulphides and Cobalt-Rich Ferromanganese Crusts: Status and Prospects This report contains the full text of four presentations originally given at an international workshop on deep ocean mineral resources beyond the limits of national jurisdiction held in Kingston, Jamaica in June 2000. International Seabed Authority 3 The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the International Seabed Authority concerning the legal status of any country or territory or of its authorities, or concerning the delimination of its frontiers or maritime boundaries. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying or otherwise, without the prior permission of the copyright owner. Applications for such permission with a statement of purpose and extent of the reproduction should be addressed to the Secretary-General, International Seabed Authority, 14-20 Port Royal Street, Kingston, Jamaica. National Library of Jamaica Cataloguing-in-Publication Data Polymetallic massive sulphides and cobalt-rich ferromanganese crusts : status and prospects. p. : ill., maps; cm. – (ISA technical study ; no.2) Includes bibliography. ISBN: 976-610-467-0 1. Oceanography -

Region 8 Japan, Taiwan and Marianas
Appendix B – Region 8 Country and regional profiles of volcanic hazard and risk: Japan, Taiwan and Marianas S.K. Brown1, R.S.J. Sparks1, K. Mee2, C. Vye-Brown2, E.Ilyinskaya2, S.F. Jenkins1, S.C. Loughlin2* 1University of Bristol, UK; 2British Geological Survey, UK, * Full contributor list available in Appendix B Full Download This download comprises the profiles for Region 8: Japan, Taiwan and Marianas only. For the full report and all regions see Appendix B Full Download. Page numbers reflect position in the full report. The following countries are profiled here: Region 8 Japan, Taiwan, Marianas Pg.399 Japan 407 Taiwan 417 USA – Marianas Islands 423 Brown, S.K., Sparks, R.S.J., Mee, K., Vye-Brown, C., Ilyinskaya, E., Jenkins, S.F., and Loughlin, S.C. (2015) Country and regional profiles of volcanic hazard and risk. In: S.C. Loughlin, R.S.J. Sparks, S.K. Brown, S.F. Jenkins & C. Vye-Brown (eds) Global Volcanic Hazards and Risk, Cambridge: Cambridge University Press. This profile and the data therein should not be used in place of focussed assessments and information provided by local monitoring and research institutions. Region 8: Japan, Taiwan, Marianas Figure 8.1 The distribution of Holocene volcanoes through the Melanesia and Australia region. The capital cities of the constituent countries are shown. Description Region 8: Japan, Taiwan and the Marianas comprises volcanoes through the main Japanese arc, the Izu Islands, Marianas Islands and the Ryuku Islands. Taiwan is considered here, separately to China. Three countries are represented here. All are included in this regional discussion and individual country profiles are provided. -

Cretaceous Alisitos Arc, Baja California
Journal of Volcanology and Geothermal Research 149 (2006) 1–46 www.elsevier.com/locate/jvolgeores View of an intact oceanic arc, from surficial to mesozonal levels: Cretaceous Alisitos arc, Baja California Cathy Busby a,*, Benjamin Fackler Adams b, James Mattinson c, Stephen Deoreo d a Department of Geological Sciences, University of California, Santa Barbara CA 93101, USA b Interdisciplinary Sciences, Skagit Valley College, 2405 E College Way, Mt Vernon, WA 98273, USA c Department of Geological Sciences, University of California, Santa Barbara CA 93101, USA d Department of Geological Sciences, University of California, Santa Barbara, CA 93106, USA Received 29 January 2005; received in revised form 13 June 2005; accepted 19 June 2005 Abstract The Alisitos arc is an approximately 300Â30 km oceanic arc terrane that lies in the western wall of the Peninsular Ranges batholith south of the modern Agua Blanca fault zone in Baja California. We have completed detailed mapping and dating of a 50Â30 km segment of this terrane in the El Rosario to Mission San Fernando areas, as well as reconnaissance mapping and dating in the next 50Â30 km segment to the north, in the San Quintin area. We recognize two evolutionary phases in this part of the arc terrane: (I) extensional oceanic arc, characterized by intermediate to silicic explosive and effusive volcanism, culminating in caldera-forming silicic ignimbrite eruptions at the onset of arc rifting, and (II) rifted oceanic arc, characterized by mafic effusive and hydroclastic rocks and abundant dike swarms. Two types of units are widespread enough to permit tentative stratigraphic correlation across much of this 100-km-long segment of the arc: a welded dacite ignimbrite (tuff of Aguajito), and a deepwater debris-avalanche deposit.