A Role for Fenugreek in Altering the Osmoregulatory
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Botanical Nano Ozonated Erotic Oil Composition with Maximum Bioavailability for Men
MOJ Bioequivalence & Bioavailability Review Article Open Access A botanical nano ozonated erotic oil composition with maximum bioavailability for men Abstract Volume 5 Issue 1 - 2018 The present patent–pending invention relates to a topical pharmaceutical nano Awad Mansour,1 Ammar Mansour2 ozonated composition(with maximum bioavailability) for male sexual response, 1University of Akron, USA including male sexual dysfunction, such as male sexual arousal, vitality, erection 2Essraa Hospital, Jordan disorders, premature ejaculation, and sexual pain disorders, and enhancing male sexual pleasure and satisfaction of the male sexual experience as well as penis enlargement. Correspondence: Awad Mansour, University of Akron, OH, The composition for treating this problem preferably formed of a natural oil mix, USA, Tel 9622–7278278, Email [email protected] fenugreek oil, watermelon oil, ginger oil and ozonated olive oil. In particular the composition is applied on penis and testicles to enhance pleasure and delay ejaculation Received: November 25, 2017 | Published: February 07, 2018 for men during intercourse. Observed results showed excellent results for all ages of men with no adverse or side effects. Abbreviations: ED, erection dysfunction; PE, premature vasodilation, smooth muscle relaxation, etc. Australian researchers at the University of Queensland1 working with colleagues at Applied ejaculation; ASN, applied science and nutrition Science and Nutrition (ASN), a Brisbane–based company that Description of the invention specializes in scientific -

Assessing Opportunities and Threats in Kazakhstan's Wild Liquorice Root Trade
April 2021 SWEET DREAMS ASSESSING OPPORTUNITIES AND THREATS IN KAZAKHSTAN’S WILD LIQUORICE ROOT TRADE Nadejda Gemedzhieva, Artyom Khrokov, Elise Heral, Anastasiya Timoshyna JOINT REPORT ABOUT US TRAFFIC is a leading non-governmental organisation working globally on trade in wild animals and plants in the context of both biodiversity conservation and sustainable development. Reproduction of material appearing in this report requires written permission from the publisher. The designations of geographical entities in this publication, and the presentation of the material, do not imply the expression of any opinion ACKNOWLEDGEMENTS whatsoever on the part of TRAFFIC or its supporting This report was completed under a project implemented between organisations concerning the legal status of any country, territory, or area, or of its authorities, 2019-2022 by TRAFFIC and the Association for the Conservation of or concerning the delimitation of its frontiers or Biodiversity of Kazakhstan (ACBK), under the support of the Keidanren boundaries. Nature Conservation Fund (KNCF). Complementary funds were also gratefully received from Aktionsgemeinschaft Artenschutz (AGA) e.V. Lead author The project aims to reduce unsustainable commercial harvest, which Nadejda Gemedzhieva poses a threat to biodiversity conservation, and to scale up successful sustainable wild liquorice root production from which local people and Published by: nature benefit. We extend our thanks to KNCF for their support. TRAFFIC International, Cambridge, United Kingdom. During the course of this study, many individuals contributed their time, SUGGESTED CITATION expertise, original research and professional advice and the authors Gemedzhieva, N., Khrokov, A., Heral. E., Timoshyna, would like to thank the staff of the following institutions: Forestry A. -
Show Activity
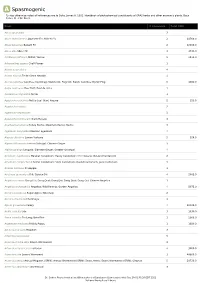
A Spasmogenic *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Abies spectabilis 3 Abies sachalinensis Japanese Fir; Shin-Yo-Yu 2 14760.0 Abies balsamea Balsam Fir 2 12000.0 Abies alba Silver-Fir 3 3736.0 Achillea millefolium Milfoil; Yarrow 5 8530.0 Achyranthes aspera Chaff Flower 1 Acinos suaveolens 4 Acinos alpinus Te de Sierra Nevada 4 Acorus calamus Calamus; Sweetflag; Sweetroot; Flagroot; Sweet Calamus; Myrtle Flag 5 1980.0 Aegle marmelos Bael fruit; Bael de India 1 Aeolanthus myriantha Ninde 1 Agastache urticifolia Nettle-Leaf Giant Hyssop 2 156.0 Agastache rugosa 2 Agastache nepetoides 3 Agastache foeniculum Giant Hyssop 3 Agathosma betulina Honey Buchu; Mountain Buchu; Buchu 3 Ageratum conyzoides Mexican ageratum 4 Aloysia citrodora Lemon Verbena 5 924.0 Alpinia officinarum Lesser Galangal; Chinese Ginger 3 Alpinia galanga Languas; Siamese Ginger; Greater Galangal 4 Amomum xanthioides Malabar Cardamom; Tavoy Cardamom; Chin Kousha; Bastard Cardamom 2 Amomum compactum Chester Cardamom; Siam Cardamom; Round Cardamom; Java Cardamom 3 Ananas comosus Pineapple 1 Anethum graveolens Dill; Garden Dill 4 2002.0 Angelica sinensis Dang Gui; Dong Quai; Dang Qui; Dang Quai; Dong Gui; Chinese Angelica 1 Angelica archangelica Angelica; Wild Parsnip; Garden Angelica 4 8073.0 Annona squamosa Sugar-Apple; Sweetsop 2 Annona cherimola Cherimoya 1 Apium graveolens Celery 4 30100.0 Aralia cordata Udo 3 1030.0 Areca catechu Pin-Lang; Betel Nut 1 2000.0 Argemone mexicana Prickly Poppy 1 1680.0 Artemisia vulgaris Mugwort 3 Artemisia salsoloides 5 Artemisia herba-alba Desert Wormwood 3 Artemisia dracunculus Tarragon 4 3000.0 Artemisia cina Levant Wormseed 1 48000.0 Artemisia annua Annual Mugwort (GRIN); Annual Wormwood (GRIN); Sweet Annie; Sweet Wormwood (GRIN); Qinghao 5 20720.0 Artemisia absinthium Wormwood 3 Dr. -

The Ancient Tale of Anise and Its Long Journey to America
For immediate release Press contact: Daniela Puglielli, Accent PR (908) 212 7846 THE ANCIENT TALE OF ANISE AND ITS LONG JOURNEY TO AMERICA New Orleans, July 2012 -- As part of the “spirited” presentations of the Tales of the Cocktail festival, Distilleria Varnelli cordially invites you to the event “Anise: The Mediterranean Treasure” on Saturday July 28, from 3:00 pm to 4:30 pm at the Queen Anne Ballroom, Hotel Monteleone in New Orleans, LA. The seminar offers a rare occasion to compare different Mediterranean anises, neat and in preparation: Varnelli, as the best Italian dry anise, ouzo, arak, raki, anisado, and anisette. Mixologist Francesco Lafranconi - winner of the TOC 2009 Best Presenter Award- and Orietta Maria Varnelli, CEO of Distilleria Varnelli S.p.a., will transport attendees through an incredible historical and cultural journey, including an exclusive tasting of anise-based Varnelli’s liqueurs and aperitifs. Renowned mixologists from London, Anistatia Miller and Jared Brown, will bring their experience to the event as well. The program will include also a short yet suggestive cultural presentation about the FIRST American Chapter of the Ordre International des Anysetiers, with Members in Medieval attire that will revive the legend and traditions of the ancient guild of Anysetiers, founded in 1263 in France. Members of the Louisiana Bailliage include Francesco Lanfranconi, who will lead the Chapter as Bailli, Tales of the Cocktail’s founders Ann and Paul Tuennerman, Liz Williams (Chair of Southern Food and Beverage Museum in NOLA), Laura and Chris McMillan of the Museum of American Cocktails – MOTAC, journalists Camper English and Brenda Maitland, mixologist Jacques Bezuidenhout and importer Paolo Domeneghetti. -

Effects of Fenugreek Seed on the Severity and Systemic Symptoms of Dysmenorrhea
Original Article Effects of Fenugreek Seed on the Severity and Systemic Symptoms of Dysmenorrhea Sima Younesy 1, Sedigheh Amiraliakbari 1*, Somayeh Esmaeili 2,3, Hamid Alavimajd 4, Soheila Nouraei 1 1- Department of Midwifery, School of Nursing and Midwifery, Shahid Beheshti University of Medical Sciences, Tehran, Iran 2- Department of Traditional Pharmacy, School of Traditional Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran 3- Traditional Medicine and Materia Medica Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran 4- Department of Biostatistics, Faculty of Paramedicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran Abstract Background: Primary dysmenorrhea is a prevalent disorder and its unfavorable ef- fects deteriorates the quality of life in many people across the world. Based on some evidence on the characteristics of fenugreek as a medical plant with anti-inflammato- ry and analgesic properties, this double-blind, randomized, placebo controlled trial was conducted. The main purpose of the study was to evaluate the effects of fenu- greek seeds on the severity of primary dysmenorrhea among students. Methods: Unmarried Students were randomly assigned to two groups who received fenugreek (n=51) or placebo (n=50). For the first 3 days of menstruation, 2−3 cap- sules containing fenugreek seed powder (900 mg) were given to the subjects three times daily for two consecutive menstrual cycles. Pain severity was evaluated using a visual analog scale and systemic symptoms were assessed using a multidimension- al verbal scale. * Corresponding Author: Sedigheh Amiraliakbari, Results: Pain severity at baseline did not differ significantly between the two Department of Midwifery, groups. Pain severity was significantly reduced in both groups after the intervention; School of Nursing and however, the fenugreek group experienced significantly larger pain reduction Midwifery, Shahid (p<0.001). -

And Liquorice
Progress in Nutrition 2018; Vol. 20, N. 4: 713-720 DOI: 110.23751/pn.v20i4.7950 © Mattioli 1885 Original article Efficiency of spearmint (Mentha spicata L.) and liquorice (Glycyrrhiza glabra L.) extracts in oxidative stability of fish oil under accelerated conditions İlknur Ucak Nigde Omer Halisdemir University, Faculty of Agricultural Sciences and Technologies, Nigde, Turkey. E-mail: [email protected] Summary. Nowadays, innumerable synthetic and naturally sourced antioxidants are used to overcome the quality problems of fish and fish products. When considering the rejection of synthetic additives by consum- ers, demand for the natural sources food supplements has increased. This study focuses on the potential use of spearmint (Mentha spicata L.) and liquorice (Glycyrrhiza glabra L.) extracts as natural antioxidant in fish oil during accelerated storage. Ethanolic (70%) extracts of spearmint and liquorice were prepared and synthetic antioxidant butylated hydroxytoluene (BHT) added into the fish oil in different concentrations (100, 500 and 1,000 ppm). The peroxide value (PV), thiobarbituric acid value (TBARS), para-anisidine value (p-Av) and UV-spectrum analyses of fish oil were evaluated during storage at 55oC. Throughout the the storage period the PV, TBARS value, p-Av, conjugated dienes (UV232) and conjugated trienes (UV270) values of spearmint and liquorice extracts added samples were significantly lower than the control and BHT added samples. Ac- cording to results of the study, 500 and 1,000 ppm concentrations of spearmint and liquorice extracts were more effective in prevention of lipid oxidation in fish oil. The results revail that spearmint and liquorice ex- tracts could be a natural antioxidant source for oxidative stability of fish oil. -

FAQ Fenugreek Seed for Increasing Milk Supply
FAQ-FENUGREEK INFORMATION Fenugreek Seed for Increasing Milk Supply By Kelly Bonyata, IBCLC EFFECT ON MILK PRODUCTION Fenugreek (Trigonella foenum-graecum L.) appears to be the herb that is most often used to increase milk supply. It has been reported to be an excellent galactagogue for some mothers, and has been used as such for centuries. The few studies that have been done have had mixed results [Swafford 2000, Reeder -pharmaceutical methods of increasing milk supply should be tried first, as there can be significant side effects from both herbal remedie2011, Turkyılmazs and prescription 2011] . Keep medications in mind thatused in to almost increase all milkcases, supply. non See the Academy of Breastfeeding Medicine’s protocol #9 on the use of galactogogues. Mothers generally notice an increase in production 24-72 hours after starting the herb, but it can take two weeks for others to see a change. Some mothers do not see a change in milk production when taking fenugreek. Dosages of less than 3500 mg per DAY have been reported to produce no effect in many women. One way reported to determine if you’re taking the correct dosage is to slowly increase the amount of fenugreek until your sweat and urine begin to smell like maple syrup. If you’re having problems with any side effects, discontinue use and consider alternative methods of increasing milk supply. Fenugreek has been used either short-term to boost milk supply or long-term to augment supply and/or pumping yields. There are no studies indicating problems with long-term usage. -

Nóinín Herbal Products Our Operating Ethos Is to Create Products That Are Single Herb Teas: As Beneficial As Possible
ORGANIC HERB TEAS Nóinín Herbal Products Our operating ethos is to create products that are Single Herb Teas: as beneficial as possible. We use only the highest Chamomile, Mint, Raspberry, Nettle, Yarrow, Sage, quality ingredients, organic wherever possible, from Birch etc. trusted and reliable sources. Many other teas available on request All the plants used are grown organically by ourselves using the biodynamic method, harvested in Composite Teas: the locality away from sources of pollution, or, in Echinacea comp. Tea “Soothing” some cases, purchased from reputable organic Contains: Elderflower, Chamomile, Calendula, Wild suppliers. Pansy, Nettle, Burdock Root, Cleavers, Echinacea Root, Liquorice, Lemon Balm We take great care with the formulation and manufacture of every product. By making small Evening Herb Tea “Relaxing” batches we ensure that products are as fresh as Contains: Valerian Root, Lemon Balm, Lavender , possible. Our products enjoy endorsement from Chamomile, Lime Blossoms massage therapists and herbal practitioners. Customer comments are unanimously favourable Herb Tea Mixture “Harmonising” and we trust your experience will be similarly Contains: Lemon Balm, Mint, Nettles, Blackberry, positive. Raspberry Leaves, Lemongrass, Calendula Flowers, Yarrow ORDERING INFORMATION Orders will be sent by post at the cheapest postal Lemon Dream Tea “Refreshing” rates unless otherwise instructed. Contains: Lemon Balm, Lemongrass, Lemon Verbena, Red Hibiscus Flowers, Rose Hips Please contact for shipping cost within Ireland and for -

Effect of Mint and Fenugreek and Mixture on Production and Immunity of Broilers
Journal of Kerbala University , Vol. 12 No.2 Scientific . 2014 EFFECT OF MINT AND FENUGREEK AND MIXTURE ON PRODUCTION AND IMMUNITY OF BROILERS تأثير النعناع والحلبة وخليطهما على اﻷداء اﻹنتاجي والمناعي لفروج اللحم Assist.Lec. Ali .R .Abed/Veterinary Medicine College- Kerbala University Assist.Lec. Fateh .O .Kadhim / Veterinary Medicine College- Kerbala University Abstract : This study conducted to determine the effect of dietary mint, fenugreek and combination on productive and immune parameters of broiler chicks (Ross308).A total of 80 broiler chicks of one day old have been reared for 35 day under good hygienic condition .The chicks were randomly divided into four groups :Treatment 1 (Mint 1%) ,Treatment 2 (fenugreek 1% ) ,Treatment 3 (Mint 1%+ fenugreek 1%) & Treatment 4(Control), each of one have 2o chicks with 2 replicate for each treatment .the addition of dry leaves of Mint and fenugreek started at one day until end of experiment, while the control group given the standard feed only .the productive parameters are measured weekly, and immune status of chicks are measured at 21 and 35 day by ELISA technique. The results have appeared that there is an improvement in performance and immunity traits for all treated groups if compared with the control group. However, the chicks feed with 1% mint and 1% fenugreek performed better than those fed with others concerning live body weight and body weight gain. with regards to feed consumption, the first treatment that supplemented with 1% mint achieved better one. the second treatment that supplemented with 1% fenugreek recorded high antibody titter against Newcastle disease virus and Gumboro disease virus at 21 and 35 day of broilers age . -

Study of the Anti-Inflammatory Activity
Pharmacology STUDY OF THE ANTI-IINFLAMMATORY ACTIVITY OF SOME MEDICINAL EDIBLE PLANTS GROWING IN EGYPT N. M. AMMAR* S. Y. AL OKBI** D. A. MOHAMED** SUMMARY: Several edible plants are used in traditional medicine for the treatment of inflammatory condi- tions. The anti-inflammatory activity of some bioactive fractions isolated from the seeds of Trigonella foenum groecum, L., the roots of Glycyrrhiza glabra, L. and the fruits of Coriandrum sativum, L. were determined using the carragenan induced oedema method in comparison with two reference synthetic anti-inflammatory drugs. The petroleum ether and the aqueous methanolic extract of fenugreek and liquorice as well as the whole pow- dered fruit of coriander in a dose of 200 mg/kg exhibited a significant reduction in the volume of inflammation with variable degreeses. The same active dose from the three plants also inhibited prostaglandins E2 levels in a range of 55-64%. Chromatographic fractionation of the bioactive components revealed the isolation of unsatu- rated fatty acids and flavonoids in the three plants, while saponins were only present in fenugreek and liquorice. The anti-inflammatory activity may be attributed to the presence of the forementioned bioactive compounds. Key Words: Anti-inflammatory, medicinal, edible plants. INTRODUCTION The severe side effects of steroidal and nons- L., family Leguminosae, (Fenugreek) and the fruits of teroidal anti-inflammatory drugs evoked us to search Coriandrum sativum L., family Umbelliferae (Coriander). for new anti-inflammatory agents from natural botanical The anti-inflammatory activity of liquorice has been sources which may have minimal drawbacks. studied and still current research is being carried out by Our review of 'folk medicine' indicated that many some authors (1-2). -

Medication: Trazodone (Desyrel) 50 Mg
Trazodone COMPLEX CHRONIC DISEASES PROGRAM Medication Handout Date: May 15, 2018 Medication: Trazodone 50 mg What is trazodone: Trazodone is an antidepressant that is now used for insomnia; it helps with both falling asleep and staying asleep. Expected Benefit: As a sleep aid, you should notice a benefit on the first night within about 30 minutes of taking the medication. Watch for possible side effects: This list of side effects is important for you to be aware of however, it is also important to remember that not all side effects happen to everyone. If you have problems with these side effects talk with your doctor or pharmacist: Hangover effect (drowsiness that continues after waking up in the morning) Dizziness Dry mouth Headache Nausea Stopping the medication: Stopping trazodone is not usually a problem as there is no withdrawal effect you are taking it regularly at higher doses than prescribed below. Please ask your doctor or pharmacist before stopping the medication. Rebound insomnia is not usually a problem which makes trazodone a good option for taking a sleeping aid as needed How to use this medication: Take this medication with or without food Dosing Schedule: Start with 12.5 mg (¼ tablet) or 25 mg (½ tablet) at bedtime Increase the dose by ¼ or ½ a tablet every night until: o You can fall asleep, and/or o Stay asleep The usual effective dose is 50 – 150 mg at bedtime (1 – 3 tablets) Do not continue increasing the dose if you experience a hangover effect Talk with your doctor if you are still having problems with sleep, as you may need a different medication. -

Bulk Numerical Codes
BBulkulk DepartmentDepartment CCodesodes BBEANSEANS GGRAINSRAINS Adzuki Beans, Organic 1200 Barley, Pearled, Organic 1300 Fava Beans 1201 Buckwheat, Raw, Organic 1302 Black Beans 1202 Buckwheat, Kasha, Organic 1303 Black Beans, Organic 1203 Polenta, Organic 1304 Black Eyed Peas, Organic 1204 Couscous, Organic 1306 Red Chili Beans, Organic 1205 Couscous, Whole Wheat, Organic 1307 Farro, Pearled 1308 Garbanzo Beans, Organic 1206 KAMUT ® Khorasan Wheat, Organic 1309 Lima Beans, Large 1207 Millet, Organic 1311 Great Northern Beans, Organic 1208 Popcorn, Yellow, Organic 1313 Lentils, Pardina 1210 Quinoa, Organic 1314 Mung Beans, Split, Organic 1211 Quinoa, Organic Red, Non-GMO 1315 Lentils, French Green, Organic 1212 Popcorn, White, Organic 1317 Lentils, Green, Organic 1213 Rye, Organic 1329 Lentils, Red, Organic 1214 Spelt, Organic 1330 Lentils, Black Beluga 1215 Wheat, Hard Red, Organic 1331 Lima Beans, Organic 1216 Wheat, Soft White, Organic 1332 Mung Beans, Organic 1217 Wheat, Hard White, Organic 1333 Navy Beans, Organic 1218 Wheat, Bulgar, Organic, Non-GMO 1334 Split Peas, Green, Organic 1219 Corn, Whole Yellow, Organic 1337 Popcorn, Multicolor, Organic 1338 Split Peas, Green 1220 Oats, Raw, Organic 1340 Split Peas, Yellow, Organic 1221 Oats, Steel Cut, Organic 1341 Pinto Beans, Organic 1222 Couscous, Israeli 1342 Pinto Beans 1223 Lentils, Harvest Gold, Organic 1224 RRICEICE Soy Beans, Organic 1225 Arborio, Organic 1250 13 Bean Soup Mix 1226 Brown Basmati, Non-GMO 1251 Kidney Beans, Dark, Organic 1227 Brown Basmati, Organic 1252 Cannelini