Mercury Bioaccumulation in Estuarine Food Webs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download File
Hazard Mitigation Technical Assistance Program Contract No. EMW-2000-CO-0247 Task Order 415 Hurricane Katrina Rapid Response Wind Water Line (WWL) Data Collection – Louisiana FEMA-1603-DR-LA Final Report June 9, 2006 Submitted to: Federal Emergency Management Agency Region IV Atlanta, GA Prepared by: URS Group, Inc. 200 Orchard Ridge Drive Suite 101 Gaithersburg, MD 20878 FOR PUBLIC RELEASE HMTAP Task Order 415 June 9, 2006 Table of Contents Abbreviations and Acronyms------------------------------------------------------------------------------- iii Glossary of Terms -------------------------------------------------------------------------------------------- iv Background------------------------------------------------------------------------------------------------------1 Overview of Impacts in Louisiana--------------------------------------------------------------------------3 Purpose---------------------------------------------------------------------------------------------------------11 Methodology---------------------------------------------------------------------------------------------------15 Findings and Observations --------------------------------------------------------------------------------25 Conclusion -----------------------------------------------------------------------------------------------------31 List of Figures Figure 1: Hurricane Katrina Storm Track-----------------------------------------------------------------1 Figure 2: FEMA-1603-DR-LA Disaster Declaration ---------------------------------------------------5 -

Multivariate Analyses of Water Quality Dynamics Over Four Decades in the Barataria Basin, Mississippi Delta
water Article Multivariate Analyses of Water Quality Dynamics Over Four Decades in the Barataria Basin, Mississippi Delta John W. Day 1, Bin Li 2 , Brian D. Marx 2, Dongran Zhao 2 and Robert R. Lane 3,* 1 Department of Oceanography and Coastal Sciences, Louisiana State University, Baton Rouge, LA 70803, USA; [email protected] 2 Department of Experimental Statistics, Louisiana State University, Baton Rouge, LA 70803, USA; [email protected] (B.L.); [email protected] (B.D.M.); [email protected] (D.Z.) 3 Comite Resources, PO Box 66596, Baton Rouge, LA 70896, USA * Correspondence: [email protected] Received: 13 October 2020; Accepted: 5 November 2020; Published: 10 November 2020 Abstract: Here we examine a combined dataset of water quality dynamics in the Barataria Basin, Louisiana based on transect studies from 1977 to 1978 (Seaton) and from 1994 to 2016. The Davis Pond river diversion into Lake Cataouatche began discharging Mississippi River water into the mid-basin in 2005, and so the later dataset was divided in Pre- and Post-diversion periods. The stations from these three datasets (Seaton, Pre- and Post-diversion) were combined into eleven station groupings for statistical analysis that included ANOVA and principal component analysis. In addition, Trophic State Index (TSI) scores were calculated for each grouping during the three time periods. Lake Cataouatche changed the most with the opening of the Davis Pond river diversion, becoming clearer and less eutrophic with addition of river water, which passed through a large wetland area where sediments were retained before entering the lake. The TSI results for the Seaton re-analysis were very similar to the original analysis and to that of the Pre- and Post-diversion datasets, indicating that the trophic status of the basin waters has remained relatively unchanged. -

Fairfield Strategic Plan
Fairfield Strategic Plan WESTBANK, JEFFERSON PARISH, LOUISIANA Prepared For: Regional Planning Commission for Jefferson, Orleans, Plaquemines, St. Bernard, St. Tammany, and Tangipahoa Parishes Jefferson Parish RPC Project H.010170JP DOTD Project No. H.010170 August 2015 Fairfield Strategic Plan Westbank, Jefferson Parish, LA Prepared By and Project Team Leads: Walter Brooks, Executive Director Meredith Soniat, Sustainability Planner and Project Manager New Orleans Regional Planning Commission 10 Veterans Memorial Boulevard New Orleans, LA 70124 P: 504-483-8500 Terri Wilkinson, Ph.D., AICP, Planning Director Jefferson Parish Government 1221 Elmwood Park Blvd., Suite 601 Jefferson, LA 70123 P: 504-736-6320 Frank Liang, P.E., PTOE, Vice President Ross Liner, AICP, PTP, Senior Project Manager Digital Engineering 527 West Esplanade Avenue, Suite 200 Kenner, LA 70065 P: 504-468-6129 Michael Lauer, AICP, Principal Planning Works, LLC 8900 State Line Road, Suite 406 Leawood, Kansas 66206 P: 913-341-8800 Dana Nunez Brown, PLA, ASLA, AICP Dana Brown & Associates, Inc. 1836 Valence Street New Orleans, LA 70115 P: 504-345-2639 8/26/2015 Fairfield Strategic Plan Westbank, Jefferson Parish, LA Table of Contents Chapter 1: Plan Overview ................................................................................................................... 1 1.1 Project Description .................................................................................................................. 1 1.2 Scope of Work .............................................................................................................................. -

Shedding Light on Our Estuary & Our Economy
Shedding Light on Our Estuary & Our Economy Environmental Indicators in the Barataria-Terrebonne Estuary System 2010 1 Courtesy of Guy Fanguy Friends: The Barataria-Terrebonne National Estuary Program’s first environmental indicators report, Healthy Estuary, Healthy Economy, Healthy Communities... Environmental Indicators in the Barataria-Terrebonne Estuary System: 2002, provided a valuable overview of the environmental health of the Barataria-Terrebonne Estuary System (BTES), and remains one of the Program’s most popular products. It is therefore a great pleasure for me to introduce an update to this report. The general format remains the same. A series of ten focus questions that residents of the BTES commonly have posed to the BTNEP Partnership is used to organize the environmental indicators presented in this report. The indicators under each focus question represent some of the BTES’ vital signs. They tell us how our estuary is doing and establish an associational link to how the BTNEP Partnership’s restoration efforts are working. The devastation brought to the BTES—our home—by the 2005 and 2008 hurricane seasons only emphasizes how important the health of our estuary is to the safety and sustainability of our communities. Post-storm analysis has shown that intact, healthy wetlands can protect homes and businesses from hurricane surge much better than degraded wetlands. The horrific experience of Hurricanes Katrina and Rita highlights the real tragedy for BTES residents and for the nation: the fact that this region is being lost to the Gulf of Mexico at an extraordinary rate. Our land loss problem will only make our communities, businesses, and entire infrastructure increasingly vulnerable to future hurricanes. -

Water Area Changes in Southeastern Louisiana After Hurricanes Katrina and Rita Detected with Landsat Thematic Mapper Satellite Imagery
Water Area Changes in Southeastern Louisiana After Hurricanes Katrina and Rita Detected with Landsat Thematic Mapper Satellite Imagery Multiple scenes of Landsat Thematic Mapper (TM) satellite imagery were peppered by marsh balls and other debris, were present throughout the acquired from the USGS Earth Resources Observation and Science (EROS) area. Over ninety percent of the new water area appearing after the hurri- Data Center to identify water area changes in southeastern coastal Louisi- canes in Breton Sound basin occurred within the 2001 fresh and intermedi- ana after Hurricanes Katrina and Rita (landfalls Aug. 29 and Sept. 24, 2005, ate marsh communities classified in 2001 (Chabreck and others, White Kitchen, Louisiana respectively). Identification of new water areas relied on visual assessments unpub.data, 2001). Comparison of Marsh Shears Before and After Hurricanes Katrina and Rita (Landsat 5 Thematic Mapper Satellite Imagery) of imagery, image classification to determine land-water areas by date, and Hammond spatial comparison of the resultant classified land-water datasets to iden- The more mineral-rich brackish and saline marshes appeared to have fared November 7, 2004 October 25, 2005 White Kitchen, Louisiana W E W E est a est a Marsh Shears After Hurricanes Katrina and Rita tify changes in water area after the hurricanes. Complete coverage of better, as indicated by the lack of large new water areas in central Breton s s t t P P P P earl e earl e southeast Louisiana was obtained by acquiring two adjacent, north-south Sound basin and the Bayou Biloxi marsh. Fringing shoreline erosion, as well a a r r R l R l image paths, one covering two-thirds and the other covering the remain- as the formation of small ponds, was observed in the brackish-saline iv R iv R P i P i Slidell er v er v Shear h e Slidell h e ing one-third of the eastern deltaic plain. -

Deepwater Horizon/Mississippi Canyon 252 Oil Spill Report on Submerged Aquatic Vegetation Residing in Jean Lafitte National Historic Park and Preserve
DRAFT DDDeeeeeepppwwwaaattteeerrr HHHooorrriiizzzooonnn///MMMiiissssssiiissssssiiippppppiii CCCaaannyyyooonnn 222555222 OOOiiilll SSSpppiiillllll RRReeepppooorrrttt ooonnn SSSuuubbbmmmeeerrrgggeeeddd AAAqqquuuaaatttiiiccc VVVeeegggeeetttaaatttiiiooonnn RRReeesssiiidddiiinnnggg iiinnn JJJeeeaaannn LLLaaafffiiitttttteee NNNaaatttiiiooonnnaaalll HHHiiissstttooorrriiiccc PPaaarrrkkk aaannnddd PPPrrreeessseeerrrvvveee NNNaaatttuuurrraaalll RRReeesssooouuurrrccceee DDDaaammmaaagggeee AAAsssssseeessssssmmmeeennnttt SSSppprrriiinnnggg aaannnddd FFFaaallllll 222000111111 RRReeepppooorrrttt Prepared for: The National Park Service Environmental Quality Division P.O. Box 25287 Denver, CO 80225-0287 and Submerged Aquatic Vegetation Technical Working Group February 2012 DRAFT Deepwater Horizon/Mississippi Canyon 252 Oil Spill Report on Submerged Aquatic Vegetation Residing in Jean Lafitte National Historic Park and Preserve Natural Resource Damage Assessment Spring and Fall 2011 Report Prepared for: The National Park Service Environmental Quality Division P.O. Box 25287 Denver, CO 80225-0287 and Submerged Aquatic Vegetation Technical Working Group Prepared by: Weston Solutions, Inc. 2433 Impala Drive Carlsbad, California 92010 February 2012 MC 252 Oil Spill – Jean Lafitte National Historic Park and Preserve Submerged Aquatic Vegetation NRDA Spring 2011 Draft Report February 2012 TABLE OF CONTENTS Executive Summary................................................................................................................... 5 1.0 INTRODUCTION ............................................................................................................. -

LUMCON News LUMCON News
A Publication by the Louisiana Universities Marine Consortium LUMCONLUMCON NewsNews Winter 2005 Volume 5, Number 1 JASON Expedition Visits South Louisiana in Search of Disappearing Wetlands Program developed by Dr. Robert Ballard, discoverer of the H.M.S. Titanic, focuses its attention on educating young students about coastal landloss Ask Amy McDowell or Taylor Landry, wrapped up filming for their live broad- JASON Expeditions filmed thirty live seventh grade students from Montegut casts that brought the importance, com- interactive broadcasts January 31– Middle School, to tell you why people plexity and fragility of south Louisiana’s February 5, 2005, at LUMCON’s should learn about Louisiana’s wetlands wetlands to over 33,000 teachers and 1.7 DeFelice Marine Center in Cocodrie, and without pause they will flood you million students worldwide. Included LUMCON’s Field Laboratory in Port with answers. Fourchon, and the Jean Lafitte National Historic Park and Preserve, Barataria “They’re like disappearing,” said Preserve. Live transmissions are just McDowell. “They won’t be here for the one part of JASON’s hands-on next generation. People need to pass on standards-based science curriculum for their importance.” fourth–ninth grade teachers and stu- dents that has focused on a different “It’s a home for animals,” said Landry. scientific location each year for the past “And it’s our home too. It’s where we sixteen years. Using the latest broadcast trap, hunt and fish. In five to ten years, technology, JASON Expeditions have will the land still be here? We need a lot virtually transported students across Student Argonaut Valerie Wilson,armed with a of people to help.” sediment corer, explains to Expedition viewers the globe from the depths of the how to use the device to take a sample of the hydrothermal vents in the Guaymas Help, in the form of education, is exactly marsh soil. -

Water Resources of Jefferson Parish, Louisiana
Prepared in cooperation with the Louisiana Department of Transportation and Development Water Resources of Jefferson Parish, Louisiana Introduction 7.16 Mgal/d from groundwater sources1 (table 1). Power generation accounted for about 91 percent of the total water This fact sheet presents a brief overview of groundwater withdrawn. Other water-use categories included public supply, and surface-water resources in Jefferson Parish, Louisiana industrial, rural domestic, livestock, and general irrigation (table (fig. 1). Information on the availability, use, and quality of 2). Water-use data collected at 5-year intervals from 1960 to water from groundwater and surface-water sources in the parish 2010 (fig. 2) indicated that water withdrawals peaked at about is discussed. Previously published reports (see References 1,600 Mgal/d during 1975 and 1980 in Jefferson Parish. The Cited section) and data stored in the U.S. Geological Survey’s large increase in surface-water withdrawals from 1970 to 1975 is National Water Information System (http://waterdata.usgs.gov/ primarily attributable to withdrawals for power generation. nwis) are the primary sources of this information. In 2010, about 817 million gallons per day (Mgal/d) of 1Tabulation of withdrawals in the text and tables may produce different totals water were withdrawn in Jefferson Parish, approximately because of rounding; nonrounded numbers are used for calculation of total 810 Mgal/d from surface-water sources and approximately withdrawals. 90°10' 90°00' 89°50' 90°10' 90°00' Lake Pontchartrain 30°10' Area enlarged 10 30°05' PARISH PARISH PARISH Lake PARISH Jf-178 ORLEANS JEFFERSON PontchartrainPontchartrain JEFFERSON 47 A ORLEANS 90 Jf-178 A PARISH Jf-186 Intracoastal Waterway Metairie Intracoastal Waterway 30°00' Kenner 610 Jf-186 510 30°00' 610 MMissis ississip Jf-156 isissisip M pi New Orleans Jf-156 ppi ST. -

Capacity of Freshwater Marsh to Process Nutrients in Diverted
Louisiana State University LSU Digital Commons LSU Master's Theses Graduate School 2004 Capacity of freshwater marsh to process nutrients in diverted Mississippi River water Craig Bonya' Johnson Louisiana State University and Agricultural and Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_theses Recommended Citation Johnson, Craig Bonya', "Capacity of freshwater marsh to process nutrients in diverted Mississippi River water" (2004). LSU Master's Theses. 862. https://digitalcommons.lsu.edu/gradschool_theses/862 This Thesis is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Master's Theses by an authorized graduate school editor of LSU Digital Commons. For more information, please contact [email protected]. CAPACITY OF FRESHWATER MARSH TO PROCESS NUTRIENTS IN DIVERTED MISSISSIPPI RIVER WATER A Thesis Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Masters of Science in The Department of Agronomy and Environmental Management by Craig Bonya’ Johnson B.S., Alcorn State University, 2002 December 2004 DEDICATION To my parents, Carl and Bobbie Johnson who have always been there through all of my dilemmas to guide, uphold, encourage, protect and most of all understand me. God has blessed me with two loving parents, who have worked hard to mold me into the individual that I am today. I’m blessed to have parents like mine as models for my life. I could not have made it this far without your motivation and dedication to my success. -

Barataria Basin
LOUISIANA DEPARTMENT OF WILDLIFE & FISHERIES OFFICE OF FISHERIES INLAND FISHERIES SECTION PART VI -A WATERBODY MANAGEMENT PLAN SERIES BARATARIA BASIN LAKE HISTORY & MANAGEMENT ISSUES 1 CHRONOLOGY April 2014 - Prepared by Tim Ruth, Biologist Manager, District 8 Gary Vitrano, Biologist Manager, District 8 August 2015 – Update by Gary Vitrano, Biologist Manager, District 8 August 2017 – Update by Gary Vitrano, Biologist Manager, District 8 Matthew Duplessis, Biologist Supervisor, District 8 December 2019 – Update by Gary Vitrano, Biologist Manager, District 8 Matthew Duplessis, Biologist Supervisor, District 8 Jonathan Winslow, Biologist 3, District 8 2 TABLE OF CONTENTS WATERBODY HISTORY .......................................................................................................................................................................... 4 GENERAL INFORMATION .................................................................................................................................................................. 4 Major Waterbodies: ............................................................................................................................................................................... 4 Parishes Located: ................................................................................................................................................................................... 4 AUTHORITY .......................................................................................................................................................................................... -

Downloaded from the USGS Website (
water Article Assessing Chlorophyll a Spatiotemporal Patterns Combining In Situ Continuous Fluorometry Measurements and Landsat 8/OLI Data across the Barataria Basin (Louisiana, USA) Ivan A. Vargas-Lopez 1,* , Victor H. Rivera-Monroy 1,* , John W. Day 1, Julie Whitbeck 2 , Kanchan Maiti 1 , Christopher J. Madden 3 and Armando Trasviña-Castro 4 1 Department of Oceanography and Coastal Sciences, College of the Coast and Environment, Louisiana State University, Baton Rouge, LA 70803, USA; [email protected] (J.W.D.); [email protected] (K.M.) 2 Jean Lafitte National Historical Park and Preserve, National Park Service, 419 Decatur St New Orleans, LA 70130, USA; [email protected] 3 Everglades Research Division, South Florida Water Management District, 8894 Belvedere Road, West Palm Beach, FL 33411, USA; [email protected] 4 Centro de Investigación Científica y de Educación Superior de Ensenada (CICESE), Unidad La Paz, La Paz, Baja California Sur 23050, Mexico; [email protected] * Correspondence: [email protected] (I.A.V.-L.); [email protected] (V.H.R.-M.) Abstract: The acquisition of reliable and accurate data to assess environmental changes over large spatial scales is one of the main limitations to determine the impact of eutrophication, and the effectiveness of management strategies in coastal systems. Here, we used a continuous in situ Citation: Vargas-Lopez, I.A.; Chl-a fluorometry sensor and L8/OLI satellite data to develop an algorithm and map Chl-a spatial Rivera-Monroy, V.H.; Day, J.W.; distribution to assess the impact of freshwater diversions and associated high nutrient loading rates in Whitbeck, J.; Maiti, K.; Madden, C.J.; the Barataria Basin (BB) complex, a coastal system in the northern Gulf of Mexico. -
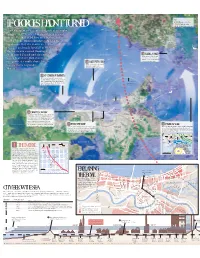
NOLA.Com: Hurricane Center
UNDER KEY: Surge water flow Wind direction IF GEORGES HADN’T TURNED Model hurricane track New Orleans most recently dodged catastrophic MANDEVILLE flooding in 1998, when Hurricane Georges cut across the Gulf of Mexico on a beeline to the mouth of the Mississippi River. As half the SLIDELL population fled, the storm veered to Pearl the east and made landfall in Mississippi. River The hurricane caused flooding in St. Bernard Parish and also pushed Lake Pontchartrain 3 SLIDELL SOAKED Large parts of Slidell would waves from Lake Pontchartrain be inundated, but the waters would recede rapidly with no up against its south shore levees, 4 LAKE LEVEES HOLD levees to contain them. Winds on the west side would leaving many to ponder: push water against the What if? hurricane-protection levees. Rigolets 5 ST. CHARLES SUBMERGED Here, water in the lake would reach heights of 3-8 feet above normal, spilling into wetlands and towns in St. Charles Approximate and St. John parishes. The water would diameter of be deepest near the river levees. hurricane’s eye Chef Menteur Pass LAPLACE EASTERN No flooding NEW ORLEANS at UNO sippi River sis is Intracoastal Waterway M KENNER Over 8 feet Over 8 feet METAIRIE Over 8 feet NORCO 4-8 feet Lake Mississippi River-Gulf Outlet Borgne 4-8 feet 6 GRAVITY’S GATEWAY NEW ORLEANS Relentless winds from the stalled hurricane 9TH WARD push a dome of water 14 feet above sea level at the levee. The model says that water Dry land on ST. BERNARD would top low levees and floodwalls and HARAHAN Metairie Ridge move east into Jefferson and Orleans Less than 4 feet PARISH parishes.