Oral Submucous Fibrosis: Etiology, Pathogenesis, and Future Research R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Use of Biologic Agents in the Treatment of Oral Lesions Due to Pemphigus and Behçet's Disease: a Systematic Review
Davis GE, Sarandev G, Vaughan AT, Al-Eryani K, Enciso R. The Use of Biologic Agents in the Treatment of Oral Lesions due to Pemphigus and Behçet’s Disease: A Systematic Review. J Anesthesiol & Pain Therapy. 2020;1(1):14-23 Systematic Review Open Access The Use of Biologic Agents in the Treatment of Oral Lesions due to Pemphigus and Behçet’s Disease: A Systematic Review Gerald E. Davis II1,2, George Sarandev1, Alexander T. Vaughan1, Kamal Al-Eryani3, Reyes Enciso4* 1Advanced graduate, Master of Science Program in Orofacial Pain and Oral Medicine, Herman Ostrow School of Dentistry of USC, Los Angeles, California, USA 2Assistant Dean of Academic Affairs, Assistant Professor, Restorative Dentistry, Meharry Medical College, School of Dentistry, Nashville, Tennessee, USA 3Assistant Professor of Clinical Dentistry, Division of Periodontology, Dental Hygiene & Diagnostic Sciences, Herman Ostrow School of Dentistry of USC, Los Angeles, California, USA 4Associate Professor (Instructional), Division of Dental Public Health and Pediatric Dentistry, Herman Ostrow School of Dentistry of USC, Los Angeles, California, USA Article Info Abstract Article Notes Background: Current treatments for pemphigus and Behçet’s disease, such Received: : March 11, 2019 as corticosteroids, have long-term serious adverse effects. Accepted: : April 29, 2020 Objective: The objective of this systematic review was to evaluate the *Correspondence: efficacy of biologic agents (biopharmaceuticals manufactured via a biological *Dr. Reyes Enciso, Associate Professor (Instructional), Division source) on the treatment of intraoral lesions associated with pemphigus and of Dental Public Health and Pediatric Dentistry, Herman Ostrow Behçet’s disease compared to glucocorticoids or placebo. School of Dentistry of USC, Los Angeles, California, USA; Email: [email protected]. -

Rebamipide to Manage Stomatopyrosis in Oral Submucous Fibrosis 1Joanna Baptist, 2Shrijana Shakya, 3Ravikiran Ongole
JCDP Rebamipide to Manage Stomatopyrosis10.5005/jp-journals-10024-1972 in Oral Submucous Fibrosis ORIGINAL RESEARCH Rebamipide to Manage Stomatopyrosis in Oral Submucous Fibrosis 1Joanna Baptist, 2Shrijana Shakya, 3Ravikiran Ongole ABSTRACT Source of support: Nil Introduction: Oral submucous fibrosis (OSF) causes progres- Conflict of interest: None sive debilitating symptoms, such as oral burning sensation (sto- matopyrosis) and limited mouth opening. The standard of care INTRODUCTION (SOC) protocol includes habit cessation, intralesional steroid and hyaluronidase injections, and mouth opening exercises. The Oral submucous fibrosis (OSF) is commonly seen in objective of the study was to evaluate efficacy of rebamipide the Indian subcontinent affecting individuals of all age in alleviating burning sensation of the oral mucosa in OSF in groups. It is a potentially malignant disorder caused comparison with SOC intralesional steroid injections. almost exclusively by the use of smokeless form of Materials and methods: Twenty OSF patients were divided into tobacco products. The malignant transformation rates two groups [rebamipide (100 mg TID for 21 days) and betametha- vary from 3 to 19%.1,2 sone (4 mg/mL biweekly for 4 weeks)] of 10 each by random Oral submucous fibrosis causes progressive debilitat- sampling. Burning sensation was assessed every week for 1 month. Burning sensation scores were analyzed using repeated ing symptoms affecting the oral cavity, such as burning measures analysis of variance (ANOVA) and paired t-test. sensation, loss of cheek elasticity, restricted tongue move- Results: Change in burning sensation score was significant ments, and limited mouth opening. Oral submucous (p < 0.05) in the first four visits. However, score between the fibrosis is an irreversible condition and the management 4th and 5th visit was not statistically significant (p > 0.05). -
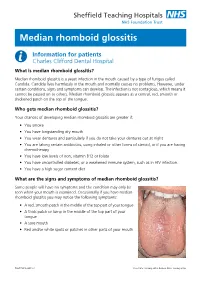
Median Rhomboid Glossitis
Median rhomboid glossitis Information for patients Charles Clifford Dental Hospital What is median rhomboid glossitis? Median rhomboid glossitis is a yeast infection in the mouth caused by a type of fungus called Candida. Candida lives harmlessly in the mouth and normally causes no problems. However, under certain conditions, signs and symptoms can develop. The infection is not contagious, which means it cannot be passed on to others. Median rhomboid glossitis appears as a central, red, smooth or thickened patch on the top of the tongue. Who gets median rhomboid glossitis? Your chances of developing median rhomboid glossitis are greater if: • You smoke • You have longstanding dry mouth • You wear dentures and particularly if you do not take your dentures out at night • You are taking certain antibiotics, using inhaled or other forms of steroid, or if you are having chemotherapy • You have low levels of iron, vitamin B12 or folate • You have uncontrolled diabetes, or a weakened immune system, such as in HIV infection. • You have a high sugar content diet What are the signs and symptoms of median rhomboid glossitis? Some people will have no symptoms and the condition may only be seen when your mouth is examined. Occasionally if you have median rhomboid glossitis you may notice the following symptoms: • A red, smooth patch in the middle of the top part of your tongue • A thick patch or lump in the middle of the top part of your tongue • A sore mouth • Red and/or white spots or patches in other parts of your mouth PD6779-PIL2645 v4 Issue Date: January 2019. -

Zeroing in on the Cause of Your Patient's Facial Pain
Feras Ghazal, DDS; Mohammed Ahmad, Zeroing in on the cause MD; Hussein Elrawy, DDS; Tamer Said, MD Department of Oral Health of your patient's facial pain (Drs. Ghazal and Elrawy) and Department of Family Medicine/Geriatrics (Drs. Ahmad and Said), The overlapping characteristics of facial pain can make it MetroHealth Medical Center, Cleveland, Ohio difficult to pinpoint the cause. This article, with a handy at-a-glance table, can help. [email protected] The authors reported no potential conflict of interest relevant to this article. acial pain is a common complaint: Up to 22% of adults PracticE in the United States experience orofacial pain during recommendationS F any 6-month period.1 Yet this type of pain can be dif- › Advise patients who have a ficult to diagnose due to the many structures of the face and temporomandibular mouth, pain referral patterns, and insufficient diagnostic tools. disorder that in addition to Specifically, extraoral facial pain can be the result of tem- taking their medication as poromandibular disorders, neuropathic disorders, vascular prescribed, they should limit disorders, or atypical causes, whereas facial pain stemming activities that require moving their jaw, modify their diet, from inside the mouth can have a dental or nondental cause and minimize stress; they (FIGURE). Overlapping characteristics can make it difficult to may require physical therapy distinguish these disorders. To help you to better diagnose and and therapeutic exercises. C manage facial pain, we describe the most common causes and underlying pathological processes. › Consider prescribing a tricyclic antidepressant for patients with persistent idiopathic facial pain. C Extraoral facial pain Extraoral pain refers to the pain that occurs on the face out- 2-15 Strength of recommendation (SoR) side of the oral cavity. -

Management of Oral Submucous Fibrosis – an Update
European Journal of Molecular & Clinical Medicine ISSN 2515-8260 Volume 07, Issue 5, 2020 Management Of Oral Submucous Fibrosis – An Update Aishwarya V1; Dr. A. Amudhan2, Dr. A. Amudhan Professor, Dept. of Oral Medicine and Radiology, Sree Balaji Dental College and Hospital, Bharath Institute of Higher Education and Research, Chennai. 1. Undergraduate student, Sree Balaji Dental College and Hospital, Bharath Institute of Higher Education and Research, Chennai. 2. Professor, Dept of Oral Medicine and Radiology, Sree Balaji Dental College and Hospital, Bharath Institute of Higher Education and Research, Chennai. Professor, Dept. of Oral Medicine and Radiology, Sree Balaji Dental College and Hospital, Bharath Institute of Higher Education and Research, Chennai. Email ID: [email protected] ABSTRACT: Oral submucous fibrosis (OSF) is an insidious, chronic, progressive, debilitating disease. It is mostly prevalent in the South-east Asian countries. Areca nut chewing usually causes the condition. The hallmark of the disease being sub mucosal fibrosis that affects most parts of the oral cavity, pharynx and upper third of the oesophagus and its clinical presentation depends on the stage of the disease at detection. As the disease has a spectrum of presentation, the management differs with the various stages of the disease. This article reviews the various medical management techniques of oral submucous fibrosis. KEYWORDS: Arecanut; Etiopathogenesis; Management; Oral submucous fibrosis INTRODUCTION: Oral submucous fibrosis was first described -

Caudal Stomatitis and Other Autoimmune Oral Disease
Caudal Stomatitis and Other Autoimmune Oral Disease Yuck, no practitioner wants to deal with these cases… But treating these will benefit your patients Autoimmune Oral Disease Multiple expressions of over response of the immune system Most common are; Caudal Stomatitis Chronic Ulcerative Paradental Stomatitis Juvenile Periodontitis Caudal Stomatitis Primarily in feline patients Hallmarks in history: Oral pain Dysphagia Ptyalism Vocalizing Distinct halitosis Diagnosis of Caudal Stomatitis Primarily a diagnosis of history and oral evaluation Oral evaluation hallmarks Palatitis Glossitis Mucositis PALATOGLOSSAL FOLD PROLIFERATION, ULCERATION Other useful diagnostic tools Hypergammaglobulinemia Bartonella titer (this is of questionable clinical relevance) Histopathology almost always shows lymphoplasmacytic infiltrate with mild to moderate fibrosis Some neutrophilic infiltrate is common WHEN IN DOUBT, SUBMIT INCISIONAL BIOPSY Pathogenesis Well…This is really up for grabs. Thought to be auto-antibodies directed at the periodontal ligament There are certainly multiple etiologies and much needs to be elucidated Probable Caudal Stomatitis Unlikely Caudal Stomatitis OK, this is the big one THERAPY OF CAUDAL STOMATITIS Divide therapy into acute therapy directed at return to eating and definitive therapy directed at long term analgesia and return to function THERE IS NO CURE, WE ARE TREATING SYMPTOMS Acute Therapy 1. In anorexic, painful cats a. Analgesics 1. Opioids 2. Non-steroidal anti-inflammatories 3. Oral antibiotics -

Oral Manifestations of Systemic Disease Their Clinical Practice
ARTICLE Oral manifestations of systemic disease ©corbac40/iStock/Getty Plus Images S. R. Porter,1 V. Mercadente2 and S. Fedele3 provide a succinct review of oral mucosal and salivary gland disorders that may arise as a consequence of systemic disease. While the majority of disorders of the mouth are centred upon the focus of therapy; and/or 3) the dominant cause of a lessening of the direct action of plaque, the oral tissues can be subject to change affected person’s quality of life. The oral features that an oral healthcare or damage as a consequence of disease that predominantly affects provider may witness will often be dependent upon the nature of other body systems. Such oral manifestations of systemic disease their clinical practice. For example, specialists of paediatric dentistry can be highly variable in both frequency and presentation. As and orthodontics are likely to encounter the oral features of patients lifespan increases and medical care becomes ever more complex with congenital disease while those specialties allied to disease of and effective it is likely that the numbers of individuals with adulthood may see manifestations of infectious, immunologically- oral manifestations of systemic disease will continue to rise. mediated or malignant disease. The present article aims to provide This article provides a succinct review of oral manifestations a succinct review of the oral manifestations of systemic disease of of systemic disease. It focuses upon oral mucosal and salivary patients likely to attend oral medicine services. The review will focus gland disorders that may arise as a consequence of systemic upon disorders affecting the oral mucosa and salivary glands – as disease. -

Alcohol Use and Oral Health Fact Sheet for PROVIDERS OCTOBER 2017
Alcohol Use and Oral Health Fact Sheet FOR PROVIDERS OCTOBER 2017 The Challenge… Glossitis – tongue inflammation Patients who drink alcohol regularly may experience specific problems related to their oral health and hygiene. Angular cheilitis – corners of the mouth chronically inflamed and cracked What you need to know… Candida – yeast infection • Patients who drink high amounts of alcohol daily may brush Oral Ulceration – painful round or oval less effectively than those who don’t drink alcohol, despite sores reporting similar brushing frequency. Also, impaired motor Acute Necrotizing activity can affect their ability to perform basic dental hygiene adequately.1 Ulcerative Gingivitis – infection of the gums that causes ulcers, swelling, and • Alcohol is also the most common cause of sialadenosis dead tissue in the mouth of the parotid gland. This condition causes swelling of the parotid gland and decreased secretion of saliva.2 Ways You Can Help… • Poor nutrient intake and absorption combined with decreased salivary excretion frequently can lead to glossitis, Recommend: angular cheilitis, candida infection, oral ulceration, and acute • Brushing thoroughly two times daily with a necrotizing ulcerative gingivitis (ANUG).2 fluoridated toothpaste. • A decreased immune response combined with a nutritionally • Rinse mouth with non-alcoholic mouth rinse. poor diet, poor oral hygiene, decreased salivary flow, and a • Have an oral examination and cleaning by a high incidence of smoking among these patients, provides dental professional at least two times per year. an environment conducive to rapid progression of periodontal • Regular oral exams that include a periodontal disease, dental caries and increased risk of oral thoracic evaluation and oral cancer screenings to detect cancers.2 any signs of suspicious lesions.3 • High consumption of alcohol may damage the liver and bone marrow resulting in excessive bleeding during dental treatment. -

Download Download
628 Indian Journal of Forensic Medicine & Toxicology, July-September 2021, Vol. 15, No. 3 Tongue Lesions - A Review N.Anitha1, Dharini Jayachandran2 1Reader, Department of Oral Pathology and Microbiology,2Undergraduate Student, Sree Balaji Dental College and Hospital, Bharath Institute of Higher Education and Research Abstract Tongue is a vital organ within the oral cavity that has varied function,and it may act as an index for the underlying systemic diseases.The investigation of the tongue diseases may begin with mere clinical examination .This review is to highlight the signs and symptoms of the various lesions that affects the tongue and especially to talk in brief about the benign and malignant tumours that affect the tongue along with other inherited and congenital abnormalities.Tongue lesions are categorized as tumours,infections, reactionary,congenital,developmental,acquired,autoimmune and potentially malignant disorders for easy understanding and to arrive at appropriate diagnosis.Tongue playing an important role in maintaining the harmony in the oral environment,it should be treated from diseases. Keywords: Tongue lesions,benign tumours,malignant tumours,diseases of tongue. CLASSIFICATION OF LESIONS ● Pyogenic granuloma AFFECTING THE TONGUE. ● Frictional keratosis BENIGN TUMOURS OF THE TONGUE INFECTIOUS LESIONS OF TONGUE ● Capillary hemangioma ● Oral squamous papilloma ● Fibroma ● Oral hairy leukoplakia ● Cavernous hemangioma ● Candidiasiis ● Giant cell granuloma ● Median rhomboid glossitis ● Lipoma ● Sublingual abcess ● Lymphangioma INHERITED,CONGENITAL,DEVELOPMENT ● Schwannoma AND ACQUIRED ABNORMALITIES OF TONGUE MALIGNANT TUMOURS OF TONGUE ● White sponge nevus ● Squamous cell carcinoma ● Foliate papillitis ● Veruccous carcinoma ● Angina bullosa hemorrhagica ● Non-Hodgkin’s lymphoma ● Geographic tongue TRAUMATIC/REACTIONARY LESIONS OF ● Fissured tongue THE TONGUE ● Median rhomboid glossitis ● Fibrous reactive hyperplasia ● Bifurcated/tetrafurcated tongue ● Traumatic ulcer Indian Journal of Forensic Medicine & Toxicology, July-September 2021, Vol. -

Tobacco Induced Oral Keratosis. Oral Sub-Mucous Fibrosis. Nicotine Stomatitis
Tobacco induced oral keratosis. Oral sub-mucous fibrosis. Nicotine stomatitis. Actinic keratosis. Actinic cheilitis Assoc. prof. Zornitsa Mihaylova, DDS, PhD Dept. of Dental, oral and maxillofacial surgery, Faculty of Dental medicine, Medical Universtity- Sofia Precancerous lesions are morphologically altered tissues that possess greater than normal tissues risk of malignant transformation. The term “potentially malignant disorders” (PMD) is broadly accepted in order to avoid terminological confusion. In significant number of cases the oral cancer is preceded by a premalignancy. On the other hand PMD may not undergo malignant transformation (especially when the bad habits are ceased and proper treatment with long-term follow up have been conducted). The following risk factors may play a significant role in the development of PMD and cancer: tobacco smoking, smokeless tobacco, betel quid, alcohol consumption (the combination of smoking and alcohol significantly increases the risk of malignant transformation), oral HPV infection, radiation, vitamin deficiency, bacterial infections, immunosuppression and immunodeficiency, drugs, poor oral hygiene, chronic trauma. It is well established that the effects of the etiologic factors may vary depending on the geographic region, the lifestyle and the habits of the population. Tobacco induced oral keratosis There are three types of smokeless tobacco: dry snuff, moist snuff and chewing tobacco. Smokeless tobacco is mainly used by young males. The long-term/chronic smokeless tobacco use causes local alterations of the oral structures due to the significant nicotine absorption. Some of the most common oral changes related to smokeless tobacco are oral mucosa lesions, periodontal disease and dental caries. Clinically asymptomatic white lesions of the oral mucosa are identified. -

Paraneoplastic Pemphigus with Clinical Features of Lichen Planus Associated with Low-Grade B Cell Lymphoma
Report Paraneoplastic pemphigus with clinical features of lichen planus associated with low-grade B cell lymphoma Sónia Coelho, MD, José Pedro Reis, MD, Oscar Tellechea, MD, PhD, Américo Figueiredo, MD, PhD, and Martin Black, MD, PhD From the Department of Dermatology, Abstract University Hospital, Coimbra, Portugal, St Background Neoplasia-induced lichen planus is described as a cell-mediated reaction to John’s Institute of Dermatology, St Thomas’ unknown epithelial antigens. Paraneoplastic pemphigus (PNP), characterized by the presence Hospital, London, UK of a specific array of autoantibodies, probably represents a different form of presentation of the Correspondence same autoimmune syndrome where the mucocutaneous expression depends on the dominant Sónia Coelho pathologic mechanism. Clínica de Dermatologia, Hospital da Methods The authors report a case of PNP with predominant lichen planus-like lesions and Universidade review the relevant literature. We observed a 74-year-old female with vesico-bullous, erosive, P.3000–075 Coimbra target-shaped and flat papular lichenoid lesions on the lower legs, palms and soles, evolving for Portugal E-mail: [email protected] 3 weeks. Histopathology revealed a lichenoid dermatitis. Direct immunofluorescence showed C3 deposition around keratinocytes and epidermal IgG intranuclear deposition. Indirect immunofluorescence revealed circulating IgG with intercellular staining on rat bladder substrate. Immunoblotting demonstrated bands of 130, 190, 210 and 250 kDa antigens. A pararenal B cell lymphoma was found. Results Oral corticotherapy with 40 mg prednisolone daily was initiated with a good cutaneous response. Four months later, cyclophosphamide (50 mg/day) was introduced because of a discrete enlargement of the pararenal mass. The patient died on the seventh month of follow up as a result of respiratory insufficiency. -

Cardiovascular Drugs-Induced Oral Toxicities: a Murky Area to Be Revisited and Illuminated
Pharmacological Research 102 (2015) 81–89 Contents lists available at ScienceDirect Pharmacological Research j ournal homepage: www.elsevier.com/locate/yphrs Review Cardiovascular drugs-induced oral toxicities: A murky area to be revisited and illuminated a, b b Pitchai Balakumar ∗, Muthu Kavitha , Suresh Nanditha a Pharmacology Unit, Faculty of Pharmacy, AIMST University, Semeling, 08100 Bedong, Malaysia b Faculty of Dentistry, AIMST University, 08100 Bedong, Malaysia a r t i c l e i n f o a b s t r a c t Article history: Oral health is an imperative part of overall human health. Oral disorders are often unreported, but are Received 20 July 2015 highly troublesome to human health in a long-standing situation. A strong association exists between Received in revised form 22 August 2015 cardiovascular drugs and oral adverse effects. Indeed, several cardiovascular drugs employed clinically Accepted 8 September 2015 have been reported to cause oral adverse effects such as xerostomia, oral lichen planus, angioedema, Available online 25 September 2015 aphthae, dysgeusia, gingival enlargement, scalded mouth syndrome, cheilitis, glossitis and so forth. Oral complications might in turn worsen the cardiovascular disease condition as some reports suggest an Keywords: adverse correlation between periodontal oral disease pathogenesis and cardiovascular disease. These are Cardiovascular drugs certainly important to be understood for a better use of cardiovascular medicines and control of associated Oral adverse effects oral adverse effects. This review sheds lights on the oral adverse effects pertaining to the clinical use of Dry mouth Angioedema cardiovascular drugs. Above and beyond, an adverse correlation between oral disease and cardiovascular Dysgeusia disease has been discussed.