Facial Nerve Palsy 7 James M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Facial Nerve Paralysis Due to Chronic Otitis Media: Prognosis in Restoration of Facial Function After Surgical Intervention
http://dx.doi.org/10.3349/ymj.2012.53.3.642 Original Article pISSN: 0513-5796, eISSN: 1976-2437 Yonsei Med J 53(3):642-648, 2012 Facial Nerve Paralysis due to Chronic Otitis Media: Prognosis in Restoration of Facial Function after Surgical Intervention Jin Kim,1* Gu-Hyun Jung,1* See-Young Park,1 and Won Sang Lee2 1Department of Otorhinolaryngology, Inje University College of Medicine, Goyang; 2Department of Otorhinolaryngology, Yonsei University College of Medicine, Seoul, Korea. Received: December 21, 2010 Purpose: Facial paralysis is an uncommon but significant complication of chronic Revised: June 16, 2011 otitis media (COM). Surgical eradication of the disease is the most viable way to Accepted: July 6, 2011 overcome facial paralysis therefrom. In an effort to guide treatment of this rare Corresponding author: Dr. Won-Sang Lee, complication, we analyzed the prognosis of facial function after surgical treatment. Department of Otorhinolaryngology, Materials and Methods: A total of 3435 patients with COM, who underwent Yonsei University College of Medicine, 50 Yonsei-ro, Seodaemun-gu, various otologic surgeries throughout a period of 20 years, were analyzed retro- Seoul 120-752, Korea. spectively. Forty six patients (1.33%) had facial nerve paralysis caused by COM. Tel: 82-2-2228-3606, Fax: 82-2-393-0580 We analyzed prognostic factors including delay of surgery, the extent of disease, E-mail: [email protected] presence or absence of cholesteatoma and the type of surgery affecting surgical outcomes. Results: Surgical intervention had a good effect on the restoration of *Jin Kim and Gu-Hyun Jung contributed equally to this work. -

The Impact of Misdiagnosing Bell's Palsy As Acute Stroke
ORIGINAL RESEARCH ClinicalClinical Medicine Medicine 2019 2017 Vol Vol 19, 17, No No 5: 6:494–8 494–8 T h e i m p a c t o f m i s d i a g n o s i n g B e l l ’ s p a l s y a s a c u t e s t r o k e Authors: I s u r u I n d u r u w a , A N e g i n H o l l a n d , B R o s a l i n d G r e g o r y C a n d K a y v a n K h a d j o o i D Idiopathic Bell’s palsy can lead to a serious and, sometimes For many clinicians, acute stroke remains a concerning permanently, disfiguring and emotionally challenging facial diagnosis in patients presenting with facial palsy, but there are palsy. Early diagnosis and treatment with corticosteroids are key characteristics which facilitate differentiation of the two important, as they significantly improve recovery rates. Bell’s conditions, often without the need for further investigations. Our palsy is a benign condition that should be diagnosed and study aimed to explore whether clinicians could diagnose Bell’s ABSTRACT managed in primary care. Patients who self-present to the palsy in patients presenting with facial palsy at initial assessment emergency department should be managed and discharged and, if not, how often they sought a specialist opinion, as well as to without needing admission. -

Facial Paralysis
FACIAL PARALYSIS What is facial paralysis? Facial paralysis is caused by damage to the facial nerve, which controls the muscles of the face. Facial paraly- sis can occur with problems in the brain or with problems to the nerve after it has exited the brain. What are the symptoms of facial paralysis? Facial paralysis is manifested by loss of the normal blink reflex.Affected animals cannot protect their eyes ap- propriately, and they often withdraw their eyeballs into the socket as a reflex because they cannot blink. Facial paralysis can cause drooping of the face on one side and drooling. There can also be decreased tear produc- tion in the eyeball, resulting in dry eye. What causes facial paralysis? In the majority of cases, an underlying cause is not identified (idiopathic facial paralysis). Facial paralysis has been linked with low thyroid level. In some cases, facial paralysis is seen concurrently with problems in the vestibular system (system of balance). This may reflect a problem in the inner ear or in the brain. If the prob- lem is inside the brain, possible causes include cancer, infection, inflammation, and stroke. There are usually additional neurologic signs in animals with brain disease (e.g. difficulty walking, change in level of alertness, abnormalities with other cranial nerves). How is facial paralysis diagnosed? A full neurological exam is necessary to determine whether the symptoms reflect a problem inside or outside of the brain. If the problem is thought to be inside the brain, an MRI +/- spinal fluid analysis is recommended. If the problem is thought to be out- side of the brain, ruling out hypothyroidism is recommended. -

Chapter 105 – Brain and Cranial Nerve Disorders Episode Overview 1
CrackCast Show Notes – Brain and Cranial Nerve Disorders – August 2017 www.canadiem.org/crackcast Chapter 105 – Brain and Cranial Nerve Disorders Episode Overview 1. List the name, function and pathologic features of the cranial nerves 2. List 6 differential diagnosis for facial pain / trigeminal neuralgia 3. Trigeminal neuralgia - describe its diagnosis and management 4. Facial nerve paralysis: List 6 differential diagnoses for facial (CN VII) paralysis 5. Describe the facial weakness deficit associated with Bell’s Palsy and list 3 other associated symptoms 6. List 4 components of treatment of Bell’s Palsy, and list the symptoms that would make you suspect Lyme Disease 7. Differentiate between herpes zoster ophthalmicus and herpes zoster oticus 8. Describe the symptoms of vestibular schwannoma and diagnostic test of choice 9. What is the pathophysiology of diabetic mononeuropathy, and which nerves are usually involved? 10. What is Uhthoff's phenomenon? 11. List 10 clinical symptoms of Multiple Sclerosis and describe its diagnosis. Describe the typical CSF findings in a patient with MS. 12. Describe the management of an acute MS exacerbation and the usual management of a patient with relapsing-remitting MS who presents with a flare Wisecracks: 1. Cerebral Venous Thrombosis Bounceback: What are the key pearls? a. List four risk factors for cerebral venous thrombosis. b. What CT findings may be seen in cerebral venous thrombosis? What is the most common CT finding? 2. Which cranial nerve is most commonly injured in trauma? 3. What syndrome is associated with bilateral acoustic neuromas? 4. How is diabetic mononeuropathy treated? Rosen’s in Perspective What are three things I can guarantee you feel a little queasy when pimped about? Well we’ve got you covered here for Cranial Nerve problems, Cerebral Venous Thrombosis and Multiple Sclerosis. -

Facial Palsy – a Case Report
Shawna Rekshmy D’dharan et al /J. Pharm. Sci. & Res. Vol. 8(10), 2016, 1206-1209 Facial Palsy – A Case Report Shawna Rekshmy D’dharan Intern, Saveetha Dental College Dr.M.P.Santhosh Kumar * Reader, Department Of Oral and Maxillofacial Surgery Saveetha Dental College and Hospital,Velappanchavadi, Chennai Tamilnadu 600077, India Abstract Facial nerve paralysis (FNP) is the most common cranial nerve disorders and it results in a characteristic facial distortion that is determined in part by the nerves branches involved. We report a case of 54-year-old female patient who came to department of oral and Maxillofacial Surgery with right hemifacial palsy since 7 years. On clinical examination, there was lack of movement of the right forehead and eyebrows, involuntary blinking of the right eye, inability to close the right eye completely and hyperkinesia of the right cheek. After series of investigations, no definitive etiology could be traced out, hence considered as unilateral bell’s palsy of the right side. Patient has been taking vitamin B complex once daily for the past one year and reported with an improvement of symptoms, hence no other interventions were made to treat this condition. In this article, we discuss the differential diagnosis of facial nerve paralysis, etiology, clinical features and treatment modalities for bell’s palsy. Keywords-Facial palsy, Bell’s Palsy, Facial nerve, Hemifacial paralysis, Unilateral facial paralysis INTRODUCTION sudden, with facial muscle weakness progressing over Facial nerve paralysis is classified as central type or hours to days. peripheral type, depending on the level of nerve injury. Bell’s palsy is diagnosed only by exclusion of all other Central type results in paralysis of the lower part of the possible causes. -
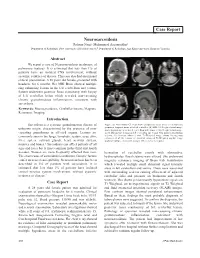
Neurosarcoidosis Case Report
Case Report Neurosarcoidosis Rohana Naqi,1 Muhammad Azeemuddin2 Department of Radiology, Dow University of Health Sciences,1 Department of Radiology, Aga Khan University Hospital,2 Karachi. Abstract We report a case of Neurosarcoidosis in absence of pulmonary features. It is estimated that less than 1% of patients have an isolated CNS involvement, without systemic evidence of disease. This case also had an unusual clinical presentation. A 28 years old female, presented with headache for 6 months. Her MRI Brain showed multiple ring enhancing lesions in the left cerebellum and vermis. Patient underwent posterior fossa craniotomy with biopsy of left cerebellar lesion which revealed non-caseating chronic granulomatous inflammation, consistent with sarcoidosis. Keywords: Neurosarcoidosis, Cerebellar lesions, Magnetic Resonance Imaging. Introduction Sarcoidosis is a systemic granulomatous disease of Figure: (a) Non-contrast CT Scan showed hypodense areas in the cerebellum and prominent temporal horns of lateral ventricles. (b) MRI, T1-Weighted axial image: unknown origin, characterized by the presence of non- shows hypointense areas in left cerebellum and vermis. (c) T2-Weighted axial image: caseating granulomas in affected organs. Lesions are iso to hyperintense lesions in left cerebellum and vermis with marked surrounding commonly seen in the lungs, lymphatic system, eyes, skin, oedema. (d) Contrast enhanced axial T1-Weighted image: shows heterogenous enhancement of the lesions. (e) Contrast enhanced T1-Weighted sagittal image liver, spleen, salivary glands, heart, nervous system, showing multiple enhancing lesions in left cerebellar hemisphere. muscles and bones.1 Sarcoidosis can affect patients of all ages and races but is most common in the third and fourth decades. Women are more frequently affected than men. -

Ramsay Hunt Syndrome - Type II
Case Report Ramsay hunt syndrome - Type II Ravneet Ravinder Verma1, Ravinder Verma2* 1Ex Senior Resident, 2Senior ENT Consultant Surgeon, 1All India Institute of Medical Sciences, Delhi, 2Verma Hospital and Research Centre, Gujral Nagar, Jalandhar, Punjab India *Corresponding Author: Ravinder Verma Email: [email protected] Abstract Ramsay Hunt syndrome is the second most common cause of facial palsy. At least three separate neurological syndromes carry the name of Ramsay Hunt syndrome (RHS), their only connection being that they were all first described by James Ramsay Hunt. Ramsay Hunt syndrome (RHS) type 1 is a rare and nebulous entity that has alternatively been called dyssynergia cerebellaris myoclonica, dyssynergia cerebellaris progressiva, dentatorubral degeneration, or Ramsay Hunt cerebellar syndrome. Ramsay Hunt syndrome (RHS) type 2 is a disorder that is caused by the reactivation of preexisting herpes zoster virus in a nerve cell bundle (the geniculate ganglion). Ramsay Hunt syndrome type III, a less commonly referenced condition, and a neuropathy of the deep palmar branch of the ulnar nerve. Ramsay Hunt Syndrome Type II (RHS) is a rare neurological disorder. This syndrome is caused by the varicella zoster virus (VZV), the same virus that causes chickenpox in children and shingles (herpes zoster) in adults. In cases of Ramsay- Hunt syndrome Type II, previously inactive varicella-zoster virus is reactivated and spreads to affect the facial nerves. The classic Ramsay Hunt syndrome, which always develops after a herpetic infection, also can be associated with vertigo, ipsilateral hearing loss, tinnitus, and facial paresis apart from otalgia. Magnetic resonance imaging (MRI) is a new and important tool for use in diagnosing and investigating diseases affecting the facial nerve. -

Vii Nerve Palsy — Evaluation and Management
VII NERVE PALSY VII NERVE PALSY — EVALUATION AND MANAGEMENT The eye cannot close and constantly weeps. The mouth dribbles, the speech is interfered with and mastication impaired. The delicate shades of continence are lost. Joy, happiness, sorrow, shock, surprise, all conditions have for their common expression the same blank stare. (Brunnell, 19279.) Facial nerve palsy is a devastating and readily visible nerve injury. Loss of tone and movement is disfiguring and causes a functional disability to eye closure, chewing, speech and facial expression. The prevalence of facial palsy in the general population is about 1:5 000, mak- ing it a common-enough clinical entity for all general practitioners to be familiar with.1,2 In most cases no definite aetiology is identified (idiopathic/Bell’s palsy). The diagnosis of an idiopathic palsy however can only be made by excluding all SHAMLAN K NAIDOO other potential causes of facial palsy. The routine assumption that all facial MB BCh, FCORL (SA) palsies are idiopathic means that many correctable causes, e.g. of otological ori- Consultant Otorhinolaryngologist gin, might be left too late to achieve any favourable outcome. Department of Otorhinolaryngology and ANATOMY Head and Neck Surgery The facial nerve leaves the brainstem and enters the internal auditory canal with Nelson Mandela School of Medicine the auditory nerve. Within the temporal bone it has three branches: University of KwaZulu-Natal •greater superficial petrosal nerve — to lacrimal glands and glands within Durban nasal and palatal mucosa • chorda tympani — to taste buds and anterior two-thirds of the tongue Shamlan Naidoo is a consultant otorhino- • stapedial nerve — stapedius muscle in middle ear. -

Iatrogenic Facial Nerve Palsy: Lessons to Learn Asma A, Marina M B, Mazita A, Fadzilah I, Mazlina S, Saim L
Original Article Singapore Med J 2009; 50(12) : 1154 Iatrogenic facial nerve palsy: lessons to learn Asma A, Marina M B, Mazita A, Fadzilah I, Mazlina S, Saim L ABSTRACT surgeon’s greatest fears during ear surgery. Despite Introduction: This study aims to review the technological advances, such as the introduction of the management and discuss the outcome of patients operating microscope and motorised surgical drill, and with iatrogenic facial nerve palsy. the availability of preoperating imaging, the overall risk of iatrogenic facial nerve palsy (FNP) remains Methods: 11 patients with iatrogenic facial nerve considerably high. The incidence of iatrogenic FNP palsy (FNP) were evaluated retrospectively in a associated with otology surgery has been estimated to be tertiary centre between June 1995 and September 0.6%–3.7%.(1) In revision mastoid surgery, the frequency 2008. All the cases were referred from other may be as high as 4%–10%.(2) Following ear surgery, centres. FNP may present immediately postoperation or develop with delayed onset. There may be complete paralysis or Results: Ten patients had iatrogenic immediate partial loss of function. Facial nerve exploration surgery FNP secondary to mastoidectomy and one had is often necessary to restore facial nerve functions. Department of Otorhinolaryngology, FNP secondary to superficial parotidectomy. The indications and the timing of exploration surgery Faculty of Medicine, Of the ten cases, three had concomitant Universiti Kebangsaan are sometimes controversial. In general, facial nerve Malaysia, profound sensorineural hearing loss and one had exploration by means of decompression with or without Jalan Yaacob Latif, Cheras, concomitant labyrinthine fistula. Ten patients restoration of the continuity of the nerve is performed Kuala Lumpur 56000, Malaysia underwent facial nerve exploration and one patient when there is immediate complete facial nerve injury was managed conservatively. -

Facial Nerve Paralysis: Report of Two Cases of Bell's Palsy
PEDIATRIC DENTISTRY/Copyright © 1987 by The American Academy of Pediatric Dentistry Volume 9 Number 1 Facial nerve paralysis: report of two cases of Bell’s palsy Martha Ann Keels, DDS Linwood M. Long, Jr., DDS, MS William F. Vann, Jr., DMD, MS, PhD Abstract Review of Facial Nerve Anatomy The cases of an ll-year-old white male and an 8-year-old black malewith facial paralysis are presented.Included are de- The facial (7th cranial) nerve has parasympa- scriptionsof diagnosis,clinical course,treatment, prognosis, and thetic, motor, and sensory nuclei in the pons, a part recommendationsfor future cases. of the brain stem located in the posterior cranial fossa. The fibers from the motor nucleus pass below the floor of the 4th ventricle and continue around the Facial nerve paralysis is an important cause of nucleus of the abducens (6th cranial) nerve to com- morbidity in children. Its presentation can be fright- bine with parasympathetic and sensory fibers to form ening for both the child and parents. The most com- a common nerve trunk. The nerve trunk exits the mon cause of facial nerve paralysis in children is brain and continues its lateral course through the Bell’s palsy2 Bell’s palsy is the accepted term to de- facial canal. Where the canal makes an acute bend scribe unilateral, peripheral facial nerve paralysis toward the middle ear cavity, the geniculate ganglion which2 is idiopathic with acute onset. is located. At the level of the geniculate ganglion, the Sometimes facial nerve paralysis is a sign of an greater superficial petrosal nerve arises and supplies underlying disease process. -

Surgical Treatment for Epstein-Barr Virus Otomastoiditis Complicated by Facial Nerve Paralysis: a Case Report of Two Young Brothers and Review of Literature
J Int Adv Otol 2017; 13(1): 143-6 • DOI: 10.5152/iao.2017.2788 Case Report Surgical Treatment for Epstein-Barr Virus Otomastoiditis Complicated by Facial Nerve Paralysis: A Case Report of Two Young Brothers and Review of Literature Evelien van Eeten, Hubert Faber, Dirk Kunst Department of Otolaryngology, Radboud University Medical Center, Nijmegen, Netherlands We report the case of two young brothers with Epstein–Barr virus (EBV) otomastoiditis complicated by a facial nerve paralysis. The boys, aged 7 months (patient A) and 2 years and 8 months (patient B), were diagnosed with a facial nerve paralysis House–Brackmann (HB) grade IV (A) and V (B). After unsuccessful pharmacological treatment, patient A underwent mastoidectomy and atticoantrotomy and patient B underwent a trans- mastoidal surgical decompression of the facial nerve. They recovered to HB grades I and II facial nerve palsy (FNP), respectively. Although rare and relatively unknown, EBV should be considered in the differential diagnosis of children with FNP of unknown cause. Surgical intervention may be a viable therapy with good recovery. KEYWORDS: Facial paralysis, otitis media, Epstein–Barr virus infections, surgical decompression INTRODUCTION The cause of acquired facial nerve palsy (FNP) in the pediatric population can be classified as infectious, traumatic, malignancy associated, hypertension associated, and idiopathic (Bell’s palsy) (Table 1). Toddlers and preteenagers may be at higher risk for FNP because of infectious and traumatic causes [1]. The main pathogens causing FNP in children are Borrelia burgdorferi (50%), idiopatic or Bell’s palsy (26%), otitis media (OM) (12%), varicella-zoster virus (6%), Herpes simplex virus (4%), and coxsackie (2%) [2]. -

Auriculotemporal and Greater Auricular Nerve Blocks Have Roles in Patients with Ramsay Hunt Syndrome with Trigeminal Nerve Involvement -A Report of Two Cases
Anesth Pain Med 2012; 7: 16~21 ■Case Report■ Auriculotemporal and greater auricular nerve blocks have roles in patients with Ramsay Hunt syndrome with trigeminal nerve involvement -A report of two cases- Department of Anesthesiology and Pain Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea Hyun Seung Jin, Woo-Seok Sim, and Hee-Jin Roe Ramsay Hunt syndrome (RHS) refers to herpes zoster infection of CN VIII or cervical spinal nerves are also involved in the the geniculate ganglion of the facial nerve. Cases complicated by disease process, in which symptoms and the clinical course are multicranial nerve involvement in the process of reactivation of the heavier and the prognosis worse, emphasizing the greater need virus, which are known to show virulent clinical course and worse prognosis, are not common in literature as in practice, and there for appropriate and earlier treatment. Although pain physicians has been only one reported case of suspected co-involvement of may actually encounter cases with multicranial nerve invol- the trigeminal nerve in Korean literature. Therefore, in cases of vement in RHS once in a while in practice, there has been RHS with severe rash over the face and neck, it is pertinent to give consideration to such multiple involvement in their early presen- only one such case report in Korean literature, where the tation. Facial nerve palsy and herpes related pain are the two authors finally concluded RHS was mistaken for a trigeminal worrisome complication, which could be alleviated by early treatment herpes zoster, rather than co-involement [1]. In search for with neural blockade in addition to oral medication.