Phase I Final Report USDA SBIR Bagaduce River Oyster Company
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Up and Up: River Herring in Eastern Maine
DOWNEAST FISHERIES PARTNERSHIP 2019 Up and up: River Herring in Eastern Maine PREPARED BY Anne Hayden, Medea Steinman, and Rachel Gorich This report is also available online at: downeastfisheries.org OUR PARTNERS coa.edu downeastinstitute.org mainesalmonrivers.org coastalfisheries.org mcht.org mainefarmlandtrust.org seagrant.umaine.edu manomet.org sunrisecounty.org wccog.net Contents 2 Introduction 2 Challenges for River Herring 4 What can be done? 5 What are River Herring? 6 Benefits of River Herring? 7 Managment 9 Status in 2018 11 Restoration Activities in Eastern Maine 14 Case Studies in River Herring Restoration 14 Bagaduce River 17 Patten Stream 17 Union River OUR PARTNERS 18 Narraguagus River 18 East Machias River 21 Orange River 21 Pennamaquan River 22 St. Croix River 23 Looking Ahead DOWNEAST FISHERIES parTNERSHIP / UP AND UP: RIVER HerrING IN EASTERN MAINE 1 Introduction River herring runs in eastern Maine are coming back! A string of successful projects, from the opening of the St. Croix fishway to the renewal of commercial fishing on Card Mill Stream in Franklin, have fueled growing momentum for restoration of rivers and streams, big and small. This report is an update on the status of river herring runs in eastern Maine. It describes the value of river herring and some of the many projects underway to allow river herring to swim upstream to their spawning habitat. Because river herring are a keystone species in freshwater and marine systems—and are so responsive to restoration efforts—they have become a focus of the Downeast Fisheries Partnership effort to restore fisheries in eastern Maine. -

Shellfish Harvesting Area Classification-Notification of Changes
S T A T E O F M A I N E D E P A R T M E N T O F M A R I N E R E S O U R C E S 2 1 S T A T E H O U S E S T A T I O N A U G U S T A, M A I N E 0 4 3 3 3 - 0 0 2 1 JANET T. MILLS PATRICK C. KELIHER GOVERNOR COMMISSIONER Shellfish Harvesting Area Classification-Notification of Changes October 18, 2020 This notice is in effect until repealed or replaced Ladies and Gentlemen: Under the authority of 12 M.R.S.A. § 6172; the Commissioner has made the following change to Area No. 500, Maine Coast Flood Closure: This notice reopens the Bagaduce River and from Eggemoggin Reach to Morgan Bay due to water quality returning to approved standards. Martin Pt (Friendship) to the Weskeag River, eastern Penobscot Bay, and from the Union River Bay to the Rt. 3 bridge in Trenton remain closed. All existing pollution and biotoxin closures remain in effect. The boundary descriptions of the area are as follows (struck text is being removed and underlined text is being added): Effective immediately, because of pollution from heavy rainfall, it shall be unlawful to dig, take or possess any clams, quahogs, oysters, mussels, whole or roe-on scallops from the shores, flats and waters; East of a line beginning at Martin Point (Friendship) running south to the limits of Maine territorial waters; AND south of a line beginning at the southern tip of Spaulding Island (South Thomaston) running west to the mainland shore and running east to the southern tip of Greens Island (Vinalhaven) then running northeast to the southern tip of Lane’s Island (Vinalhaven): AND east of a line beginning at Telegraph Point (North Haven) running south to Calf Point (Vinalhaven); AND east of a line beginning at Pendleton Point (Islesboro) running southwest to Crabtree Point (North Haven); AND south of a line beginning at Turtle Head (Islesboro) running southeast to Sawyer Pt (Cape Rosier); AND west of the Deer Isle Bridge (Sargentville/Deer Isle); AND south of the N. -

Survey of Hancock County, Maine Samuel Wasson
The University of Maine DigitalCommons@UMaine Maine History Documents Special Collections 1878 Survey of Hancock County, Maine Samuel Wasson Follow this and additional works at: https://digitalcommons.library.umaine.edu/mainehistory Part of the United States History Commons Repository Citation Wasson, Samuel, "Survey of Hancock County, Maine" (1878). Maine History Documents. 37. https://digitalcommons.library.umaine.edu/mainehistory/37 This Book is brought to you for free and open access by DigitalCommons@UMaine. It has been accepted for inclusion in Maine History Documents by an authorized administrator of DigitalCommons@UMaine. For more information, please contact [email protected]. SURVEY OF HANCOCK COUNTY. A SURVEY OF HANCOCK COUNTY, MAINE BY SAMIUEL WASSON. MEMBER OF STATE BOARD OK AGRICULTURE. AUGUSTA: SPRAGUE, OWEN A NASH, PRINTERS TO THE STATE. 1878. PREFACE. At the meeting of the Board of Agriculture held at Calais. a resolution was passed, urging the importance to our agri cultural literature of the publication of surveys of the differ ent counties in the State, giving brief notes of their history, industrial resources and agricultural capabilities ; and direct ing the Secretary to procure such contributions for the annual reports. In conformity with this resolution, and also as ear ning out the settled policy of the Board in this respect— evidences of which are found in the publication of similar reports in previous volumes—I give herewith a Survey of the County of Hancock, written by a gentleman who has been a member of the Board of Agriculture, uninterruptedly, from its first organization, and who is in every way well fitted for the work, which he has so well performed. -
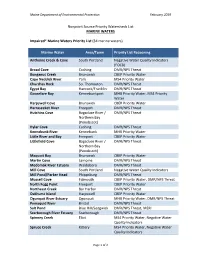
Nonpoint Source Priority Watersheds List MARINE WATERS
Maine Department of Environmental Protection February 2019 Nonpoint Source Priority Watersheds List MARINE WATERS Impaired* Marine Waters Priority List (34 marine waters) Marine Water Area/Town Priority List Reasoning Anthoine Creek & Cove South Portland Negative Water Quality Indicators (FOCB) Broad Cove Cushing DMR/NPS Threat Bunganuc Creek Brunswick CBEP Priority Water Cape Neddick River York MS4 Priority Water Churches Rock So. Thomaston DMR/NPS Threat Egypt Bay Hancock/Franklin DMR/NPS Threat Goosefare Bay Kennebunkport MHB Priority Water, MS4 Priority Water Harpswell Cove Brunswick CBEP Priority Water Harraseeket River Freeport DMR/NPS Threat Hutchins Cove Bagaduce River / DMR/NPS Threat Northern Bay (Penobscot) Hyler Cove Cushing DMR/NPS Threat Kennebunk River Kennebunk MHB Priority Water Little River and Bay Freeport CBEP Priority Water Littlefield Cove Bagaduce River / DMR/NPS Threat Northern Bay (Penobscot) Maquoit Bay Brunswick CBEP Priority Water Martin Cove Lamoine DMR/NPS Threat Medomak River Estuary Waldoboro DMR/NPS Threat Mill Cove South Portland Negative Water Quality Indicators Mill Pond/Parker Head Phippsburg DMR/NPS Threat Mussell Cove Falmouth CBEP Priority Water, DMR/NPS Threat North Fogg Point Freeport CBEP Priority Water Northeast Creek Bar Harbor DMR/NPS Threat Oakhurst Island Harpswell CBEP Priority Water Ogunquit River Estuary Ogunquit MHB Priority Water, DMR/NPS Threat Pemaquid River Bristol DMR/NPS Threat Salt Pond Blue Hill/Sedgwick DMR/NPS Threat, MERI Scarborough River Estuary Scarborough DMR/NPS Threat Spinney Creek Eliot MS4 Priority Water, Negative Water Quality Indicators Spruce Creek Kittery MS4 Priority Water, Negative Water Quality Indicators Page 1 of 2 MDEP NPS Priority Watersheds List – MARINE WATERS February 2019 Marine Water Area/Town Priority List Reasoning Spurwink River Scarborough MHB Priority Water, DMR/NPS Threat St. -
2001 Annual Report Maine Coast Heritage Trust Works
2001 Annual Report Maine Coast Heritage Trust works to conserve coastal and other lands that define Maine’s distinct landscape, protect its environment, sustain its outdoor traditions and promote the well-being of its people. Since 1970, MCHT has helped to protect permanently more than 111,000 acres including valuable wildlife habitat, farm and forest land and 275 entire coastal islands. MCHT provides conservation services to landowners, local land trusts, government agencies and communities throughout Maine. As a membership organization, MCHT is supported by individuals committed to protecting Maine’s natural beauty and resources. The Trust invites your support and involvement. On the cover: Tinker Island, Blue Hill © Sara Gray 2001 Annual Report CHRIS HAMILTON North Haven PRESIDENT’S AND CHAIRMAN’S MESSAGE In August 2001, we announced our Campaign for the Coast – a $100 million campaign that seeks to preserve the best of Maine’s coast for generations to come. Recognizing the threats posed by increasing development, we committed to accelerating our work to conserve coastal islands and archipelagos, prominent hills and meadows, shore access, community open space and intact landscape features. Campaign Chairman Richard Rockefeller inspired us to launch this ambitious effort and has stood at the helm ever since. He leads by example – guiding us with keen perception, good humor and unflagging energy. The Campaign’s success to date is due – in no small part – to his integral involvement. Already the Campaign for the Coast has expanded the horizon of land conservation in Maine, enabling protection of lands that could never before have been preserved. Among the 36 projects completed in 2001 are some of the most innovative and complex ones in MCHT’s history. -

The Bagaduce River Written by Liliana Muise
October 25 2018 The Bagaduce River Written by Liliana Muise. What is BHHT BHHT stands for Blue Hill Heritage Trust, a local east-coast nonprofit land conservation organization dedicated to saving the natural beauty of Maine and all of its animal residents. Founded in 1985 by a large group of Blue Hill Peninsula civilians, they have taken immense strides. BHHT has now protected nearly 10,000 acres of land, almost all with public access for recreational, ecological and historical practice. Educational opportunities are also available to people of all ages. From a group of school children to some curious tourists, every one has access to the lands and their beauty. Located at 157 Hinkley Ridge Rd Blue Hill, Hancock County, Maine and a website at www.bluehillheritagetrust.org, information is available to the public at any time. The BHHT’s goal or mission stated in the Blue Hill Heritage Trust’s brochure titled, From Bucksport to Stonington Hiking Trails and Public Access Points of the Greater Blur Hill Peninsula on page three: “To conserve in perpetuity land and water resources that support the long-term health and well-being of the natural and human communities on the Blue Hill Peninsula.” October 25 2018 What is a Watershed The definition of a watershed, also known as a drainage basin, is: 1 :Any area of land where precipitation (rain fall) collects and drains off into a common outlet, such as into a river, bay, or other body of water. Watersheds include all nearby streams that run down slopes, towards the shared outlet, as well as the groundwater underneath the earth’s surface. -

Maine Horseshoe Crab (Limulus Polyphemus) Spawning Surveys, 2004
Maine Horseshoe Crab (Limulus polyphemus) Spawning Surveys, 2004 Susanne Schaller1, Peter Thayer2, Sherry Hanson2, Shari LaTulippe1 and Elizabeth Solet1 March 2005 Funded by the Maine Department of Marine Resources, Maine Coastal Program, Casco Bay Estuary Project and Bar Mills Ecological. 1 Bar Mills Ecological, 71 Main Street, Buxton, Maine 04093-6101, 207.929.5490, [email protected] 2 Maine Department of Marine Resources, P. O. Box 8, West Boothbay Harbor, Maine 05475, 207.633.9500 Rev A Acknowledgements The following organizations and individuals provided support for the 2004 Maine Horseshoe Crab Surveys: Maine Coastal Program, Maine Department of Marine Resources (DMR), Friends of Taunton Bay (FTB), Bar Mills Ecological, Linda-Lee Barton, DVM, Frank and Mary Beth Dorsey; Mark, Lisa, Phoebe and Henry Herrington; Gary Blazon, The Chewonki Foundation, Friends of Casco Bay, Bagaduce Watershed Association, Bagaduce Water Watch, Thomas Point Beach and Damariscotta River Association. In addition, Mark Lazarri, DMR shared data on Boothbay Harbor sea bottom temperatures; Slade Moore, DMR, and Steve Perrin, FTB, provided information on horseshoe crab wintering habitat in Taunton Bay and 2004 temperature data; and Peter Milholland, of the Friends of Casco Bay, shared Casco Bay water temperature data. This project depends on volunteers to conduct fieldwork. Thanks go to the tagging crew which works over 40 consecutive days at Taunton Bay, Franklin: Shari LaTulippe, Elizabeth Solet, Deb Boswell Lane, Patricia Brauner, Chris Wiebusch, Ross Lane, and Phoebe Herrington. Frank Dorsey of frankstat provided statistical review, and Gary Blazon provided database assistance. Many, many volunteers conducted counts for the Surveys at field sites on Casco Bay in Cumberland, Yarmouth, Freeport, and Brunswick; at mid-coast sites in Wiscasset, Damariscotta, Nobleboro, and Sedgwick; and on Taunton Bay in Sullivan. -

2021 Alewife Trail
2021 them— striped bass, bluefish, tuna, cod, haddock, halibut, Maine’s annual spring alewife migration is inspiring. American eel, rainbow trout, brown trout, landlocked We created a map to help you find locations to salmon, smallmouth bass, pickerel, pike, white and yellow witness this natural wonder. perch, seabirds, bald eagles, osprey, great blue heron, We recommend using a mapping program like Google cormorants, seals, whales, otter, mink, fox, raccoon, Maps, with the local information included here. In many fisher, and turtles. instances you can see a fish ladder or dam in Google A single female alewife can lay 60,000-100,000 eggs, Maps, satellite view. but as few as three may survive to adulthood. Adults Alewives are anadromous (sea-run) fish that spend the make their way back to the ocean after spawning, and majority of their lives at sea but return to freshwater to many return the following spring to spawn again. spawn. Alewives (also known as river herring) are guided Commercial harvesting rights are held by towns where by their sense of smell to migrate upstream from the there are sustainable runs of alewives. The harvest ocean to rivers, streams, ponds and lakes to spawn. Runs can generate substantial income for a town, and it is generally occur between May 15 to June 15 depending on often used for the maintenance of fishways and dams. water temps and river flows. Harvested alewives are Pilgrims were taught by Native Americans to put a fish sold for lobster bait. To in the ground as fertilizer when planting corn. Those fish get a permit the town Celebrate spring were very likely alewives. -

Acadian Maine in Archaeological Perspective Alaric Faulkner
Northeast Historical Archaeology Volume 14 Article 1 1985 Acadian Maine in Archaeological Perspective Alaric Faulkner Gretchen Faulkner Follow this and additional works at: http://orb.binghamton.edu/neha Part of the Archaeological Anthropology Commons Recommended Citation Faulkner, Alaric and Faulkner, Gretchen (1985) "Acadian Maine in Archaeological Perspective," Northeast Historical Archaeology: Vol. 14 14, Article 1. https://doi.org/10.22191/neha/vol14/iss1/1 Available at: http://orb.binghamton.edu/neha/vol14/iss1/1 This Article is brought to you for free and open access by The Open Repository @ Binghamton (The ORB). It has been accepted for inclusion in Northeast Historical Archaeology by an authorized editor of The Open Repository @ Binghamton (The ORB). For more information, please contact [email protected]. Acadian Maine in Archaeological Perspective Cover Page Footnote Excavations at Fort Pentagoet were funded principally through Research Grant RS-20295-82 from the National Endowment for the Humanities. Preliminary work at both Pentagoet and Saint-Castain's Habitation was supported through the Department of the Interior, National Parks Service, and the funds administered through Maine Historic Preservation Commission. We are extremely grateful to all of those agencies and especially to Earle Shettleworth and Robert Bradley of the MHPC for seeding archaeological research on Acadian sites in Maine. Philip Perkins deserves special credit for providing laboratory and kitchen facilities in his home, and other favors during both projects. Norman Barka has graciously allowed us to study and photography the Fort La Tour collection. We extend our appreciation to Marc Lavoie, Jean Chapelot, Jorge Barrera, and many others who have shared with us their knowledge of 17th-century French material culture. -

Maine State Legislature
MAINE STATE LEGISLATURE The following document is provided by the LAW AND LEGISLATIVE DIGITAL LIBRARY at the Maine State Law and Legislative Reference Library http://legislature.maine.gov/lawlib Reproduced from scanned originals with text recognition applied (searchable text may contain some errors and/or omissions) I L.U.O. I V1 ~o. (DfVIa u <; ~~~·1 I ·-V vtutn:S I Report to the Joint Standing Committee on Marine Resources I and the Joint Standing Committee on Natural Resources I in response to Resolve Chapter 109 (LD 1528, LR 1911) I I Prepared by the Departments of Marine Resources & Environmental Protection I January 30, 2008 I. I. I I I I KF 5588 I .Z99 M25 I 2008 I I I j 1 Resolve Chapter 109 (LD 1528, LR 1911) required the Department of Inland Fisheries and Wildlife (DIFW), the Department of Marine Resources (DMR), and the Department of Environmental Protection (DEP) to : • review and update their plans for passage of native diadromous fish; • develop a proposed water quality standard that defines a fish kill; and • evaluate the processing of petitions filed with the Board of Environmental Protection (BEP) to reopen hydropower licenses. This report presents the findings and recommendations of this work for the first two matters to the Joint Standing Committees on Marine Resources and Natural Resources. The BEP is presenting its report on the petition process under separate cover. Recommendations • Implement the identified priority projects for fish passage (DMR) • Maintain the current water quality standard for protection of aquatic life (DEP) Section 1. DMR review of statewide fish passage efforts Maine’s waters are home to 12 species of native diadromous1 fishes, each of which has specific habitat requirements. -

Recognizing Achievements, Celebrating Success Appendix A
Tides of Change Across the Gulf : Chapter 7 - Recognizing Achievements, Celebrating Success Appendix A - Background Information on Groups Table of Contents Massachusetts Page Essex County Greenbelt Association Inc. .. 1 Massachusetts Bays Monitoring Program. .. 3 Massachusetts Bay Monitoring Program . 6 Massachusetts Water Resources Authority (MWRA) . .7 New England Aquarium Diver Club . 8 Tufts Centre for Conservation Medicine . .9 Woods Hole Oceanographic Institution . .11 Woods Hole Oceanographic Institution . 15 Maine Bagaduce Watershed Association . .21 Blue Hill Heritage Trust . 22 Casco Bay Estuary Project . 23 Cove Brook Watershed Council/8 Rivers Roundtable . 26 Damariscotta River Association . 27 Downeast Salmon Federation . .29 East Penobscot Bay Environmental Alliance . 31 FairPlay for Harpswell . 32 Friends of Acadia. 34 Friends of Taunton Bay . 35 Georges River Tidewater Association . 37 Island Institute . 39 Marine Environmental Research Institute (MERI)] . 41 Maine Sea Grant/Cooperative Extension marine Extension Team . 48 Quoddy Regional Land Trust . 51 Sheepscot Valley Conservation Association . 53 The Chewonki Foundation . 54 The Lobster Conservancy . .. 56 The Maine Chapter of The Nature Conservancy . 59 The Ocean Conservancy-New England Regional Office . 60 Union River Watershed Coalition. .. .62 University of Southern Maine . 63 Vinalhaven Land Trust . 64 Wells NERR Coastal Training Program . 66 New Hampshire Centre for Coastal and Ocean Mapping B University of New Hampshire . 67 Coastal Conservation Association of NH . 68 Nova Scotia Acadia Centre for Estuarine Research . .69 Bay of Fundy Ecosystem Partnership . 71 Bay of Fundy Ecosystem Partnership/Minas Basin Working Group. 73 Bedford Institute of Oceanography . 75 Centre for Water Resources Studies, Dalhousie University . 76 Clean Annapolis River Project . 77 Dr. Arthur Hines School . -

Fisheries History of the Bagaduce River
FISHERIES HISTORY OF THE BAGADUCE RIVER “The Bagaduce River defined each of the communities which flourished along its banks. Its unique geography, the depth of its waters, and the effects of the tide all contributed to the unique ways in which the river was used…No one community held the full expression of the river. The river provided opportunities for all, and each community depended upon the others to fulfill its unique role. The river was a maritime community.” --Mark Honey, Penobscot, Maine 1761-2011 “At one time the most important fishing district of eastern Maine.” – George Brown Goode, US Fish Commission Dozens of clam shell heaps along the shores of the lower Bagaduce mark places where ancestors of the Wabanaki people lived and gathered food. They steamed, roasted, and dried clams, and ventured out on the water to hunt for other fish. Within the shells are pottery fragments, tools of stone and bone, fish hooks and harpoon points. The name Bagaduce derives from the native place-name for “big tideway river,” or, in some sources, “place where there is no safe harbor,” a reference to the numerous falls and complex currents. The native people did, however, frequent the river to fish for smelt, alewives, salmon, sturgeon, tomcod, eel, and shellfish. The Bagaduce was part of a major canoe route (Minnewokun or “many directions route”) that avoided the rough seas around Cape Rosier via a portage into Walker Pond at the “Punchbowl” on Eggemoggin Reach. Many of the roads in the area follow historic Indian pathways, and the landscape is featured in several Wabanaki creation stories.