Quality Assurance Program for Anatomical Pathologists Printed Copies Are Uncontrolled
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Medical Directors Arup Medical Directors and Consulting Faculty | 2015
MEDICAL DIRECTORS ARUP MEDICAL DIRECTORS AND CONSULTING FACULTY | 2015 MAY 2015 www.aruplab.com Information in this brochure is current as of May 2015. All content is subject to change. Please contact ARUP Client Services at (800) 522-2787 with any questions or concerns. ARUP LABORATORIES ARUP Laboratories is a national clinical and anatomic pathology reference laboratory and a nonprofit enterprise of the University of Utah and its Department of Pathology. Located in Salt Lake City, Utah, ARUP offers in excess of 3,000 tests and test combinations, ranging from routine screening tests to esoteric molecular and genetic assays. Rather than competing with its clients for physician office business, ARUP chooses instead to support clients’ existing test menus by offering complex and unique tests, with accompanying consultative support, to enhance their abilities to provide local and regional laboratory services. ARUP’s clients include many of the nation’s university teaching hospitals and children’s hospitals, as well as multihospital groups, major commercial laboratories, group purchasing organizations, military and other government facilities, and major clinics. In addition, ARUP is a worldwide leader in innovative laboratory research and development, led by the efforts of the ARUP Institute for Clinical and Experimental Pathology®. Since its formation in 1984 by the Department of Pathology at the University of Utah, ARUP has founded its reputation on reliable and consistent laboratory testing and service. This simple strategy contributes significantly to client satisfaction. When ARUP conducts surveys, clients regularly rate ARUP highly and respond that they would recommend ARUP to others. As the most responsive source of quality information and knowledge, ARUP strives to be the reference laboratory of choice for community healthcare systems. -

Understanding Your Pathology Report: Benign Breast Conditions
cancer.org | 1.800.227.2345 Understanding Your Pathology Report: Benign Breast Conditions When your breast was biopsied, the samples taken were studied under the microscope by a specialized doctor with many years of training called a pathologist. The pathologist sends your doctor a report that gives a diagnosis for each sample taken. Information in this report will be used to help manage your care. The questions and answers that follow are meant to help you understand medical language you might find in the pathology report from a breast biopsy1, such as a needle biopsy or an excision biopsy. In a needle biopsy, a hollow needle is used to remove a sample of an abnormal area. An excision biopsy removes the entire abnormal area, often with some of the surrounding normal tissue. An excision biopsy is much like a type of breast-conserving surgery2 called a lumpectomy. What does it mean if my report uses any of the following terms: adenosis, sclerosing adenosis, apocrine metaplasia, cysts, columnar cell change, columnar cell hyperplasia, collagenous spherulosis, duct ectasia, columnar alteration with prominent apical snouts and secretions (CAPSS), papillomatosis, or fibrocystic changes? All of these are terms that describe benign (non-cancerous) changes that the pathologist might see under the microscope. They do not need to be treated. They are of no concern when found along with cancer. More information about many of these can be found in Non-Cancerous Breast Conditions3. What does it mean if my report says fat necrosis? Fat necrosis is a benign condition that is not linked to cancer risk. -

Simple Technique to Identify Haemosiderin in Immunoperoxidase Stained Sections
J Clin Pathol: first published as 10.1136/jcp.37.10.1190 on 1 October 1984. Downloaded from 1190 Technical methods Phosphate buffer at pH 8*0 gave the sharpest 2 Rozenszajn L, Leibovich M, Shoham D, Epstein J. The esterase staining reactions, although there was little differ- activity in megaloblasts, leukaemic and normal haemopoietic cells. Br J Haematol 1968; 14:605-19. ence at pH 7-0 or pH 7-5. As the buffer pH was 3Hayhoe FGJ, Quaglino D. Haematological cytochemistry. Edin- increased above pH 8-0 staining with both substrates burgh: Churchill Livingstone, 1980. became progressively weaker, especially above pH 4Li CY, Lam KW, Yam LT. Esterases in human leucocytes. J 9.0. Below pH 7-0 staining with a-naphthyl butyrate Histochem Cytochem 1973;21:1-12. Yam LT, Li CY, Crosby WH. Cytochemical identification of became weaker, and below pH 5*0 staining with monocytes and granulocytes. Am J Clin Pathol 1971;55:283- naphthol AS-D chloroacetate began to disappear. 90. 6 Armitage RJ, Linch DC, Worman CP, Cawley JC. The morphol- This work was supported by a Medical Research ogy and cytochemistry of human T-cell subpopulations defined by monoclonal antibodies and Fc receptors. Br J Haematol Council project grant. I thank Professor FGJ 1983;51:605-13. Hayhoe for valuable advice. References Requests for reprints to: Dr DM Swirsky, Department of Gomori G. Chloroacyl esters as histochemical substrates. J His- Haematological Medicine, University Clinical School, Hills tochem Cytochem 1953;1:469-70. Road, Cambridge CB2 2QL, England. Simple technique to identify identification of the two compounds on the same haemosiderin in slide. -
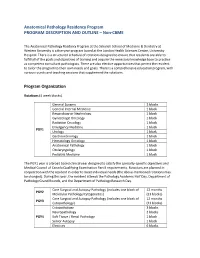
Anatomical Pathology Residency Program PROGRAM DESCRIPTION and OUTLINE – Non-CBME
Anatomical Pathology Residency Program PROGRAM DESCRIPTION AND OUTLINE – Non-CBME The Anatomical Pathology Residency Program at the Schulich School of Medicine & Dentistry at Western University is a five year program based at the London Health Sciences Centre, University Hospital. There is a structured schedule of rotations designed to ensure that residents are able to fulfill all of the goals and objectives of training and acquire the necessary knowledge base to practice as competent consultant pathologists. There are also elective opportunities that permit the resident to tailor the program to their own needs and goals. There is a comprehensive education program, with various rounds and teaching sessions that supplement the rotations. Program Organization Rotations (4 week blocks) General Surgery 2 blocks General Internal Medicine 1 block Respirology or Nephrology 1 block Gynecologic Oncology 1 block Radiation Oncology 1 block Emergency Medicine 1 block PGY1 Urology 1 block Gastroenterology 1 block Hematology Oncology 1 block Anatomical Pathology 1 block Otolaryngology 1 block Pediatric Medicine 1 block The PGY1 year is a broad based clinical year designed to satisfy the specialty-specific objectives and Medical Council of Canada Qualifying Examination Part II requirements. Rotations are planned in conjunction with the resident in order to meet individual needs (the above-mentioned rotations may be changed). During this year, the resident attends the Pathology Academic Half Day, Department of Pathology Grand Rounds, and the Department -

2019-General-Pathology-Faq.Pdf
FREQUENTLY ASKED QUESTIONS – 2019 Program: GENERAL PATHOLOGY Specialty/Field Questions: 1. a) What are some strengths about your specialty? What draws and keeps people in your specialty? • The opportunity to understand the nature of disease in an in-depth way that isn’t achieved in any other specialty. You get to practice scientific diagnostic medicine over a broad range of subjects and you have a fair degree of control over your schedule. You are very much a part of the team taking care of the patient (it just isn’t as obvious!), and this is becoming even more apparent in the era of precision medicine. • Although there is limited direct contact with patients, there is a great deal of contact with a variety of clinicians outside the laboratory as well as with physician, PhD, and technical staff colleagues within the laboratory. We get satisfaction from the interactions we have with others, and knowing that we are helping a clinician take the next step in diagnosing or treating a patient. The stereotypical image of the hermit- like pathologist who stays in their office and never talks to anyone is rapidly disappearing in today’s pathology practice. b) What are some common complaints about your specialty? • There is limited direct clinical contact – you’ll never have an office full of patients who regard you as their doctor and most pathologists have a hospital-based practice which can limit professional autonomy. • You often have to sit/stand at a microscope and/or computer a lot, so sometimes you need to get creative about staying active during the day. -

Consensus Guideline on Concordance Assessment of Image-Guided Breast Biopsies and Management of Borderline Or High-Risk Lesions
- Official Statement - Consensus Guideline on Concordance Assessment of Image-Guided Breast Biopsies and Management of Borderline or High-Risk Lesions Purpose To outline the management approach for borderline and high risk lesions identified on image-guided breast biopsy. Associated ASBrS Guidelines or Quality Measures 1. Image-Guided Percutaneous Biopsy of Palpable and Nonpalpable Breast Lesions 2. Performance and Practice Guidelines for Stereotactic Breast Procedures 3. Concordance Assessment Following Image-Guided Breast Biopsy Methods Literature review inclusive of recent randomized controlled trials evaluating the management of various borderline and high-risk lesions (including atypical hyperplasia, lobular neoplasia, papillary lesions, radial scars and complex sclerosing lesions, fibroepithelial lesions, mucocele-like lesions, spindle cell lesions, and pseudoangiomatous stromal hyperplasia [PASH]) identified on image-guided breast biopsies. This is not a complete systematic review but a comprehensive review of the modern literature on this subject. The ASBS Research Committee developed a consensus document which the ASBS Board of Directors reviewed and approved. Summary of Data Reviewed Percutaneous core needle biopsy (CNB) is the preferred, initial, minimally invasive diagnostic procedure for nonpalpable breast lesions or palpable breast masses.1 Concordance assessment of the histologic, imaging, and clinical findings determines further management. Discordance refers to the situation in which a breast CNB demonstrates benign histology, while the clinical or imaging findings are suspicious for malignancy. If there is discordance between imaging and pathology, histological evaluation is still needed. This can be accomplished either by repeat CNB, perhaps with consideration of larger gauge or vacuum- assisted device, or surgical excision.2-5 Some nonmalignant CNB findings are considered “borderline” because of their potential association with malignancy. -

2021 Anatomic & Clinical Pathology
BEAUMONT LABORATORY 2021 ANATOMIC & CLINICAL PATHOLOGY Physician Biographies Expertise BEAUMONT LABORATORY • 800-551-0488 BEAUMONT LABORATORY ANATOMIC & CLINICAL PATHOLOGY • PHYSICIAN BIOGRAPHIES Peter Millward, M.D. Mitual Amin, M.D. Chief of Clinical Pathology, Beaumont Health Interim Chair, Pathology and Laboratory Medicine, Interim Chief of Pathology Service Line, Beaumont Health Royal Oak Interim Physician Executive, Beaumont Medical Group Interim Chair, Department of Pathology and Laboratory Medicine, Oakland University William Beaumont School Interim System Medical Director, Beaumont Laboratory of Medicine Outreach Services Board certification Associate Medical Director, Blood Bank and • Anatomic and Clinical Pathology, Transfusion Medicine, Beaumont Health American Board of Pathology Board certification Additional fellowship training • Anatomic and Clinical Pathology, • Surgical Pathology American Board of Pathology Special interests Subspecialty board certification • Breast Pathology, Genitourinary Pathology, • Blood Banking and Transfusion Medicine, Gastrointestinal Pathology American Board of Pathology Lubna Alattia, M.D. Kurt D. Bernacki, M.D. Cytopathologist and Surgical Pathologist, Trenton System Medical Director, Surgical Pathology Board certification Beaumont Health • Anatomic and Clinical Pathology, Chief, Pathology Laboratory, West Bloomfield American Board of Pathology Breast Care Center Subspecialty board certification Diagnostic Lead, Pulmonary Tumor Pathology • Cytopathology, American Board of Pathology Diagnostic -

Cytopathology Surgical Pathology
CLINICAL INFORMATICS CYTOPATHOLOGY HEMATOPATHOLOGY SURGICAL PATHOLOGY This is a two-year ACGME-accredited fellowship This one-year fully accredited program This is a one-year, fully accredited fellowship This is a one-year program designed to give includes cross-disciplinary learning for physicians provides advanced training in diagnostic in hematology/hematopathology with an the fellow experience working at the junior from different medical specialties. Training cytology. The experience includes daily optional non-accredited second year faculty level. The fellowship is based at the includes specialized coursework in foundational sign-out of gynecologic and dedicated to research in hematopathology. University Hospital, with annual surgical CI as well as healthcare analytics, cybersecurity, nongynecologic specimens as well as The hematopathology fellowship includes pathology volume of 24,000 specimens. and data science. Fellows will work with the training in the performance and comprehensive training in laboratory program director to develop an individualized interpretation of fine needle aspiration hematology and interpretation of tissue Clinical duties include daily review of RUSH learning plan including foundational knowledge biopsies. Participation in conferences and biopsies performed for hematolymphoid and STAT cases, serving as first-line as well as elective opportunities (e.g., healthcare teaching of pathology residents and disorders. The accredited year of fellowship consultant to resident and student trainees, business intelligence, machine learning/artificial cytotechnology students is required. training includes core training in the clinical frozen section interpretation, organization intelligence, population/community health, hematology laboratory at University Hospital of conferences, participation in surgical bioinformatics for large scale-nucleic acid Involvement in clinical research is also and the flow cytometry, molecular pathology quality improvement activities, sequencing and clinical metabolomics, sensor encouraged. -

The Pathology of Cancer
University of Massachusetts Medical School eScholarship@UMMS Cancer Concepts: A Guidebook for the Non- Oncologist Radiation Oncology 2018-08-03 The Pathology of Cancer Chi Young Ok The University of Texas MD Anderson Cancer Center Et al. Let us know how access to this document benefits ou.y Follow this and additional works at: https://escholarship.umassmed.edu/cancer_concepts Part of the Cancer Biology Commons, Medical Education Commons, Neoplasms Commons, Oncology Commons, Pathological Conditions, Signs and Symptoms Commons, and the Pathology Commons Repository Citation Ok CY, Woda BA, Kurian E. (2018). The Pathology of Cancer. Cancer Concepts: A Guidebook for the Non- Oncologist. https://doi.org/10.7191/cancer_concepts.1023. Retrieved from https://escholarship.umassmed.edu/cancer_concepts/26 Creative Commons License This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 4.0 License. This material is brought to you by eScholarship@UMMS. It has been accepted for inclusion in Cancer Concepts: A Guidebook for the Non-Oncologist by an authorized administrator of eScholarship@UMMS. For more information, please contact [email protected]. The Pathology of Cancer Citation: Ok CY, Woda B, Kurian E. The Pathology of Cancer. In: Pieters RS, Liebmann J, eds. Chi Young Ok, MD Cancer Concepts: A Guidebook for the Non-Oncologist. Worcester, MA: University of Massachusetts Bruce Woda, MD Medical School; 2017. doi: 10.7191/cancer_concepts.1023. Elizabeth Kurian, MD This project has been funded in whole or in part with federal funds from the National Library of Medicine, National Institutes of Health, under Contract No. HHSN276201100010C with the University of Massachusetts, Worcester. -

Policy and Procedure: Infection Control Department of Anatomic Pathology, Boston Medical Center June 11 2007
Policy and Procedure: Infection Control Department of Anatomic Pathology, Boston Medical Center June 11 th 2007 Prepared by: Chris Andry, Ph.D., Administrative Director, Anatomic Pathology Gail Garvin, RN, Hospital Epidemiology Bob Burke, RN, Hospital Epidemiology Distribution: All Medical Staff, House Officers and Support Staff All laboratories at 670 Albany St., HP 2093, Newton Pavilion 3800, Autopsy Suite HB11 1 Policy and Procedure: Infection Control Department of Anatomic Pathology, Boston Medical Center Prepared by: Date reviewed: 6/11/07 Chris Andry, Ph.D., Administrative Director, Anatomic Pathology Gail Garvin, RN, Hospital Epidemiology Bob Burke, RN, Hospital Epidemiology Distribution: All Medical Staff, House Officers and Support Staff All Medical Staff, House Officers and Support Staff 670 Albany Street laboratories, Menino 2093, Newton Pavilion 3800, Autopsy Suite HB11 Role and Scope of the Department of Anatomic Pathology I. Patient Care Mission Statement The patient care mission of the department is to provide state of the art pathologic interpretation of surgical and cytopathology patient accessions, and, in the context of each patient’s clinical setting, to furnish diagnostic reports to their physicians, that are timely, complete and accurate. The department is also committed to the performance of autopsies on patients who die in this medical center in a conscientious and respectful manner and to providing expert and timely reports of autopsy findings. The goal of such reports is to provide detailed information on causes of mortality and morbidity to deceased patient’s families and attending physicians, and to contribute to the knowledge of disease and improvement of the quality of patient care. The department actively contributes to the overall mission of the Boston Medical Center: “to provide exceptional care without exception”. -

Anatomical Pathology
Timely and accurate pathology results are Pathology disciplines critical to the functioning of our entire medical system. 70% of all diagnoses are made using a pathology test. All chronic conditions require monitoring via pathology testing. Pathologists play a Pathology informs the clinical decisions of medical practitioners much wider role than just diagnosing cancer, and work across a range across the healthcare spectrum. of different specialities, in addition to anatomical pathology. These include: Given its critical role, the risks of not adequately supporting Pathologists are Indispensable to Quality Patient Care a strong national pathology system are: Chemical pathology, which deals with the entire range of disease, • Incorrect diagnoses; and encompasses detecting changes in a number of substances in • Delayed diagnoses; blood and body fluids (such as electrolytes, enzymes and proteins); • Patients receiving incorrect treatment; Forensic pathology, which seeks to investigate and define the cause • Tumours inadequately or inappropriately treated (e.g. of unexpected death; unnecessary surgery); and Genetics, which looks at chromosomes and DNA from cells to • Possible early patient death. diagnose genetic diseases; These issues may impact upon the physical, emotional and Haematology, which deals with diseases that affect the blood such as financial well-being of individual patients, their families and the anaemia, leukaemia, lymphoma, clotting or bleeding disorders as well community at large. as management of blood transfusions; Immunopathology, which deals with the diagnosis and management As the peak body representing the profession, the RCPA of conditions in which the immune system does not function properly; believes the underlying principles of a world class pathology service are: Microbiology, which deals with diseases caused by infectious agents • A commitment to patient safety and quality such as bacteria, viruses, fungi and parasites; and • A highly trained and sufficiently resourced workforce General pathology, which covers the profession as a whole. -

Pleomorphic Lipoma • Chondroid Lipoma
PATHOLOGY UPDATE: SurgicalDiagnostic Pearls for the Practicing Pathologist Friday, October 7, 2016 Aria® Resort & Casino • Las Vegas, Nevada Educational Symposia TABLE OF CONTENTS Friday, October 7, 2016 The Trouble with Fat: Diagnostic Issues in Well-Differentiated Lipomatous Tumors (John R. Goldblum, M.D.) ................ 1 Practical Approach to Melanocytic Tumor (Steven D. Billings, M.D.) .................................................................. 15 Reporting of Prostate Cancer in Needle Biopsy Specimens: Gleason Grading and More (David J. Grignon, M.D., FRCP(C)) ..................................................................... 45 Unraveling the Mesenchymal Madness in Gynecologic Tumors (Kristen A. Atkins, M.D.) ........................................ 73 REGISTER TODAY - 2017 Pathology Symposia 1 2 The Trouble With Fat: Diagnostic Issues in Well-Differentiated Lipomatous Tumors John R. Goldblum, M.D. Chairman, Department of Pathology, Cleveland Clinic Professor of Pathology, Cleveland Clinic Lerner College of Medicine Cleveland, Ohio Benign Lipomatous Tumors Lipomatous Tumors of Intermediate Malignancy • Lipoma • Angiomyolipoma • Lipoblastoma • Myelolipoma Atypical lipomatous tumor • Angiolipoma • Hibernoma (Well-differentiated liposarcoma) • Myolipoma • Spindle cell / pleomorphic lipoma • Chondroid lipoma Liposarcoma Malignant Lipomatous Tumors • Atypical lipomatous tumor (well-differentiated liposarcoma) • Dedifferentiated liposarcoma • lipoma-like • Myxoid liposarcoma • sclerosing • Round cell liposarcoma • inflammatory