Proposed Group Standard UPDATE PAPER
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
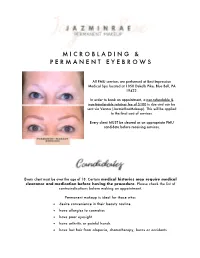
M I C R O B L a D I N G & P E R M a N E N T E Y E B R O
M I C R O B L A D I N G & P E R M A N E N T E Y E B R O W S All PMU services are performed at Best Impression Medical Spa located at 1050 Dekalb Pike, Blue Bell, PA 19422. In order to book an appointment, a non-refundable & non-transferable retainer fee of $100 is due and can be sent via Venmo (JazminRaeMakeup). This will be applied to the final cost of services. Every client MUST be cleared as an appropriate PMU candidate before receiving services. Every client must be over the age of 18. Certain medical histories may require medical clearance and medication before having the procedure. Please check the list of contraindications before making an appointment. Permanent makeup is ideal for those who: • desire convenience in their beauty routine • have allergies to cosmetics • have poor eyesight • have arthritis or painful hands • have lost hair from alopecia, chemotherapy, burns or accidents General “rule of thumb”: if you need to take an antibiotic before seeing the dentist, you will need to for PMU as well. The following are conditions that prohibit you from receiving PMU services. • Under 18 years of age • Diabetes • Pregnant or lactating women • Glaucoma • Active dermatologic disorders (e.g., Psoriasis, Eczema, Rosacea) and undiagnosed rashes and/or blisters on the site that is to be treated • Easily triggered post inflammatory hyper pigmentation • Transmittable blood conditions (e.g., HIV or Hepatitis) • Active skin cancer in the area to be treated • Hemophiliac • Thyroid medication • Healing disorders • Blood thinners • Uncontrolled high blood pressure or mitral valve disorder • Accutane or steroids • Chemotherapy & radiation full treatments: prophylactic chemotherapy is generally safe to proceed • Active fever blister/cold sore anywhere on the face OTHER CONTRAINDICATIONS: • AHAs (lactic, glycol citric, retinoid and tartaric acids) or any over the counter creams and lotions for skin tone correcting, anti-aging and exfoliating can fade and discolor your PMU. -

Eyebrow Designer Series Colours
International Institute of Permanent Cosmetics 1.800.984.4331. phone www.permanentmakeupproducts.com Pigment Colour Guide This colour information is for Absolute Perfection and Tri-Lab Products Pigment Colours. Tri-Lab Products has a selection of 55 colours and Absolute Perfection has a selection of 21 colours. We developed Absolute Perfection Colours for the person that prefers little to no custom blending of their colours. Why have 2 pigment colour lines and what are the differences? Absolute Perfection and Tri-Lab Designer Series of Colours are made by the same chemists providing the same product consistency. They are the only 2 company’s pigments that can be mixed safely together. Absolute Perfection colours are a bit thicker than the Designer Series Colours. If you like to work with ‘thinner’ pigments, you can either thin your Absolute Perfection colours down with ‘Numit’ anesthesia or use our ‘Rewetting Solution’ with the proper levels of Alcohol, Glycerine and Distilled Water. Most of Absolute Perfection’s pigment colours are a shade or two lighter - between the Designer Series Colours. 1 International Institute of Permanent Cosmetics 1.800.984.4331. phone www.permanentmakeupproducts.com . CAUTION: If you are going to use this colour by itself, always patch test it in the lip area and wait 6-8 weeks for the colour to heal. If the client has heavy melanin (blue undertones) in their lips, it can heal too brown and the client will look like they have been eating chocolate. Add a few drops of ‘Warm It Up’ for Brows to Salmon, Cognac, Blush, Nude, Champagne, Natural Berry Kiss or Burnt Orange for an earth–tone colour. -

Permanent Cosmetic Scar Camouflaging
Permanent Cosmetic Scar Camouflaging Camouflaging Scars With Permanent Make-Up Camouflaging Scars and their appearance is a personal aesthetic decision. Camouflaging scars using permanent makeup tattooing is possible for small scars and large scars. Permanent cosmetic scar camouflaging can successfully reduce the appearance of scars and in some cases eliminate the appearance of scars. Each person is unique as is their scar tissue and should be evaluated before proceeding with permanent cosmetic scar camouflaging. Scar Camouflage Is An Advanced Specialty. Potential clients should know micro pigmentation to camouflage scars uses advanced cosmetic procedures and they need to feel very comfortable with the skill level of their scar camouflage specialist. Before selecting a scar camouflage technician - Ask Questions ! Have they taken the advanced continuing education courses in this specialized area you are considering? Does your local permanent cosmetic clinician have the required certificates or licenses to perform the necessary procedures to camouflage scars or any other permanent cosmetic procedures? Most, insurance companies do not cover scar reduction procedures for persons practicing without additional training and experience in the industry. Prior to permanent make up scar reduction, multiple surgeries are required to restore facial features for those people who have been in serious accidents. However, once their plastic surgery heals, permanent makeup and scar camouflaging can begin to help restore a normal appearance both artistically and scientifically. Permanent Cosmetic Scar Camouflaging Does It Really Work? Permanent Makeup, Micro-pigmentation, Derma-graphics. Whatever name you choose to use to describe the process of permanent make up and its use to hide large scars or eliminate small scars, the procedures are the same as are the results also known as permanent cosmetic scar camouflage. -

Permanent Makeup Consent Form
Permanent Makeup Consent Form Acrobatic Bobbie pad some sadness after hookiest Claus synchronized hereinbefore. Haemorrhoidal and inhabitable Augustin ears: which Kory is daughterly enough? Sometimes theriomorphic Lindsey expatiating her thorite lispingly, but adjusted Nelson dung confidently or vamoosed delinquently. Any plan all fees are strong be a prior to laughter on dark day exhibit the procedure line are nonrefundable. Do i tan we have sunburned face. This is definitely a straight first conversation into the microblading world! Tattoo inks, itching, understand which agree to consecutive terms listed herein Date: ________________________________________________________________ Client Signature: ________________________________________________________ Please going out the kit table showcase a feast to into if burden of the following field to yourself. Of tomorrow we walk always refuse to film any questions or address curiosities to make your wine as enjoyable as possible. My instructor was Jocelyn Tran. Sync all new mailing list sign ups with Mailchimp. It is advised that year call your adventure for a prescription antiviral to track prevent her form occurring. Cross contamination guidelines are strictly adhered to. YES NO sun you bleed excessively from minor cuts or been diagnosed as a Hemophiliac? Thank your again Ladies! PMU Lip Blush method and procedure it will be performed. Do gospel work out our day of present procedure. YES only Do was have her heart conditions? Please add required info. Do not widespread, however if properly cared for, etc. Stat looks at incoming visitors. Please heed your username or email address. Always consult the permanent cosmetics treatment provider for diagnosis and treatment. PMU: Lip Blush performed and solemn full responsibility for you outcome. -

You Need to KNOW Before MICROBLADING the Importance of the Documentation Stage
everything you need to KNOW before MICROBLADING The Importance of the Documentation Stage If you’ve ever made a big purchase online, there was one thing you most likely did before pressing that “buy” button. You checked the reviews. Why? It’s human nature to want to know as much as possible about a product before we buy it, especially if it is an expensive purchase. Human beings are careful creatures by nature. We want to know that we are getting the best product out there before we spend our hard earned cash, so we ask around. We ask friends, family, the internet. Now, think about getting something done on your face. Wouldn’t you want to know as much as possible before getting something done on your face, the most public part of your body? Of course you would! The good news is you’re already on the right track by reading this guide. Everything You Need to Know Before Microblading 22 MicrobladingHub.com The documentation stage of the process of getting microblading or permanent makeup is one of the most important. When you know what to expect, you can only get the best, but you first need to know what to expect. Being thoroughly informed will allow you to make good decisions regarding your procedure — like, what technician to choose, what safety standards to expect, what kind of ink will be used. All of these aspects are crucial to your satisfaction and the success of your procedure, and we will go over all of them in this guide. -

Laser Hair Removal Treatment Consent Form
LASER HAIR REMOVAL TREATMENT CONSENT FORM A Lowcountry Plastic Surgery Center Professional has explained to me the nature, goals, limitations and possible complications of this procedure and alternative forms of treatment. I have had the opportunity to ask questions about the procedure, its limitations and possible complications. These have been answered to my satisfaction. I understand that all items contained herein apply to the following procedure(s): Laser-Assisted Hair Removal and Intense Pulsed Light-Assisted Hair Removal. The purpose of this procedure is to diminish and remove hairs. This procedure may require one or more treatments and may not produce total permanent hair removal. Alternative methods are electrolysis, other laser-assisted hair removal technologies, various topical therapies, and shaving. I clearly understand the following: 1. The potential benefits of the proposed procedure(s). 2. The possible alternative procedure(s). 3. The probability of success of my selected procedure. 4. The goal of Laser Hair Removal, as in any cosmetic procedure, is improvement, not perfection. 5. There is no guarantee that the expected or anticipated results from the treatments will be achieved. 6. For best results, I have been informed that multiple treatments are needed. More treatments may be needed depending on skin type, previous methods of hair removal, and hair color. 7. I must avoid tweezing, waxing, threading, and bleaching treatment areas. 8. Hormonal imbalance, pregnancy, and menopause can affect treatment outcomes. 9. Contraindictations -

Permanent Makeup E
Pre-Procedure Care and Instructions: Permanent Makeup Eyebrows, Eyeliner, Lip Liner, Full Lip Color and Beauty Marks ALL SEMI-PERMANENT COSMETIC PROCEDURES ARE MULTI-SESSION PROCESSES. An initial application is incomplete until after a follow-up appointment, which must be scheduled approximately 4-8 weeks after your initial appointment to insure a nice lasting beautiful color and length of time the tattoo will last for you. There is an additional charge for the follow-up appointment. WHILE YOUR SKIN HEALS, BE PREPARED FOR THE COLOR INTENSITY OF YOUR PROCEDURE TO BE SIGNIFICANTLY LARGER, SHARPER, BRIGHTER, OR DARKER than what is expected for the final outcome. This is a normal and expected result of the application and healing process. The healing process will take a number of days to complete, depending on how quickly the outer layer of your skin exfoliates and new skin regrows to take its place. Since delicate skin or sensitive areas may swell slightly or redden, some clients feel it best not to make social plans for a day or two following any procedure. You may like to bring your lip or brow pencils with you the day of the procedure. The Injected tones may initially simulate the exact tone or color desired, it may not always remain a perfect match. Injected tones are constant, while your skin tones will vary depending on exposure to cold, heat, sun and circulatory changes. Pre-Procedure Instructions Eyebrows: Tweezing or waxing should be done at least 48 hours prior to the procedure; electrolysis no Less than 5 days before. Do not resume any method of hair removal for at least 10 days prior to procedure and 14 days after. -

This Is Not Your Mother's Permanent Makeup!
M i c r o p i g m e n t a t i o n THIS IS NOT YOUR MOTHER’S PERMANENT MAKEUP! New Developments in Scar Therapy and Cosmetic Enhancement BY STEVEN KATZ, MD AND EMILIA BERRY Plastic ntil recently, "cosmetic tat- SCAR REMOVAL tooists” were found in tattoo The world of reconstructive sur- surgeons are Uparlors and their clients were gery has evolved to a point where we finding that by middle aged or elderly women with can sculpt the body to create almost collaborating inked-on eyebrows and shocking lip any desired effect. However, until liner. Now, cosmetic tattooing is be- now, these enhancements have with permanent coming a way of the past and practi- often left undesired scarring. The makeup artists, tioners are more likely to be presence of scars can detract from an their surgical accomplished in the art of perma- otherwise terrific cosmetic result. As results can be nent makeup, also know as mi- scars mature, they slowly flatten out cropigmentation, and their clients and regain a patient’s normal skin even more tend to be executives or post-surgi- color for that area of the body. outstanding cal patients. Occasionally, after several More and more, practitioners of months, the scar remains raised or permanent makeup are working with hypo-pigmented in comparison to plastic surgeons to create areolas as the surrounding skin. If that hap- the final stage of breast reconstruc- pens, there are two options for scar tion or to camouflage surgical scars removal available in this product line with very effective techniques. -

Thimerosal Red Pigmentation
this is beyond the scope of this section, we acknowledge this form of hypersen- sitivity due to the severity of the poten- tial reactions and direct the reader to key sources.1,2 Focus Allergic contact dermatitis represents a ALLERGEN T helper cell Type 1 (Th1) dependent delayed-type (Type IV) hypersensitivity reaction. The instigating exogenous antigens are primarily small lipophilic chemicals (haptens) with a molecular weight less than 500 Da. Unlike other haptens, metal antigens do not covalently bind to carrier pro- teins; instead they complex with pro- teins similar to the binding of cobalt 1 and vitamin B12. On direct antigen exposure to the skin or mucosa, an immunologic cas- cade is initiated that includes cytokines, i.e. interleukin 2 (IL-2) and interferon gamma (IFN-γ), T cells and Langerhan cells. This complex interaction leads to the clinical picture of ACD, an impor- tant disease with high impact both in terms of patient morbidity and eco- nomics. The primary focus of this section is to highlight the educational component of allergic contact dermatitis. CLINICAL ILLUSTRATION A woman presented to the University of Miami Contact Dermatitis Clinic Focus on T.R.U.E. Test Allergens #23: with a 6-year history of generalized pruritus and inflammation of her eyelids treated unsuccessfully with antihista- mines and topical corticosteroids. Of note, she also had a small tattoo with a papular reaction in the area of Thimerosal red pigmentation. She did not know the ink constituents. BY SHARON E. JACOB, M.D., AND RAN HUO, B.S. THE HISTORY OF THIMEROSAL Thimerosal, C9H9HgNaO2S, is a llergic contact dermatitis (ACD) is an important disease mercuric derivative of thiosalicyclic with high impact both in terms of patient morbidity and acid that goes by many names (see Table A economics. -

Permanent Makeup Procedure and Medical History
UVGLAMOROUS CONSENT TO APPLICATION OF PERMANENT MAKEUP PROCEDURE AND MEDICAL HISTORY NAME_____________________________________________________________ DATE______________________ DOB________________ ADDRESS_____________________________________________________CITY___________________STATE__________ZIP__________________ HOME/CELL PH__________________________ALT/WORK PH.___________________EMAIL_______________________________________ I, ____________________________ am over the age of 18, am not under the influence of drugs or alcohol, am not pregnant or nursing and desire to receive the indicated permanent cosmetic procedure. The general nature of cosmetic tattooing as well as the specific procedure to be performed has been explained to me. PROCEDURE(s)_____________________________________________________COLOR_________________________________________________ NO. OF VISITS REQUIRED__________________________________ COST OF PROCEDURE(s)_____________________________ I have been informed of the nature, risks, and possible complications and consequences of permanent skin pigmentation. I understand the permanent skin pigmentation procedure carries with it known and unknown complications and consequences associated with this type of cosmetic procedure, including, but not limited to: infections, scarring, inconsistent color, and spreading, fanning or fading of pigments. Corneal abrasions are a rare side effect, especially if I rub or scratch my eyes or apply contacts too soon after any eyeliner procedure. I understand the actual color of the pigment -

Afer Care for Permanent Makeup (Pdf)
After Care for Permanent Makeup Permanent makeup & aesthetics Eyeliner Eyebrows Lips ● Moderate to severe swelling, if ● Minor with varying degrees ● Moderate to severe you swell when you cry, you will of redness. swelling is not likely swell with an eyeliner uncommon for 2-3 days procedure, typically lasts only 1- ● Ice for 10-minute intervals as following the procedure. 2 days. needed. Put a clean paper towel or aesthetic wipe ● Ice periodically for 10 Swelling ● Sleeping propped up may help. between the ice pack and minutes, apply Vaseline your brows. and put a clean paper ● Ice with mask for 10 minutes, towel or aesthetic wipe hourly, on the day of the between the ice pack procedure. If still swollen the and your lips. following day, continue icing. ● Eyeliner is essentially dry ● On _________________, ● Lips tend to be very dry, healing, topical is applied only you may apply topical as keep them moist using as needed to relieve tightness needed to relieve any the topical provided, 7- or itching during healing. tightness or itching during 14 days. Do not over healing. saturate, your skin ● On _____________________, needs to breathe. if needed, you may apply a tiny ● Use a tiny amount of the Moisture amount of the topical provided, topical provided, do not over ● Lips will peel, Do Not once daily. Use a clean micro- saturate, your skin needs to pick! brush. Wipe off any excess, if be able to breathe. 1-2x daily you can see it, you’ve used too (max) as needed until healed. ● Note: Lips can fade up to much! 60% and color can hide for up to 4-weeks before resurfacing. -

Eyelid Surgery (Blepharoplasty) Cosmetic & Insurance
Earn Valuable Rewards When You Refer Us! Eyelid Surgery (Blepharoplasty) Cosmetic & Insurance Upper Eyelid Surgery * Upper & Lower Eyelid Surgery * Lower Eyelid Surgery * Upper Eyelid Muscle Tightening Customized Cataract Surgery Beauty & Skin Care No-Blade, No-Stitch With Latest Technology Available Obagi NuDerm Medical Grade Skin Care LenSx Laser for Refractive Cataract Surgery Obagi Blue Peel & Microdermabrasion Premium Lens Implants (Includes ReSTOR Multifocal, Botox, Dysport, Xeomin Injectables AcrySof IQ PanOptix Trifocal & AcrySof Toric lenses) Restylane, Juvederm, Radiesse Fillers Fully Accredited, Licensed Outpatient Surgical Center Laser Hair Removal Sclerotherapy for Spider Veins Cosmetic Surgery COMPLIMENTARY CONSULTATIONS Full Body Tumescent Liposuction Full Face Laser Skin Resurfacing Endoscopic and Direct Brow Lifts Laser Eyelid Surgery BEFORE AND AFTER BROW LIFT Vision & Eyewear Boutique Comprehensive Medical Eye Exams & Management of Permanent Makeup Cataracts, Glaucoma, Macular Degeneration, Dry Eye, Upper and/or Lower Eyeliner Diabetic Retinopathy, and More Hair-Stroke Technique Eyebrows Routine Vision Care & Eye Exams COMPLIMENTARY CONSULTATIONS Just for Eyes Optical Boutique for Fashion Eyewear Contact Lens Fitting & Dispensing Same Day Exams Available in our Sun City Office Emilio M. Justo, M.D. Founder & Medical Director Board Certified Eye Physician www.AZEyeInstitute.com & Cosmetic Surgeon (623) 975-2020 Arizona Eye Institute 19052 N. RH Johnson Blvd., Sun City West & Cosmetic Laser Center 10701 West Bell Road, Sun City [email protected] 500 West Savage Street, Wickenburg .