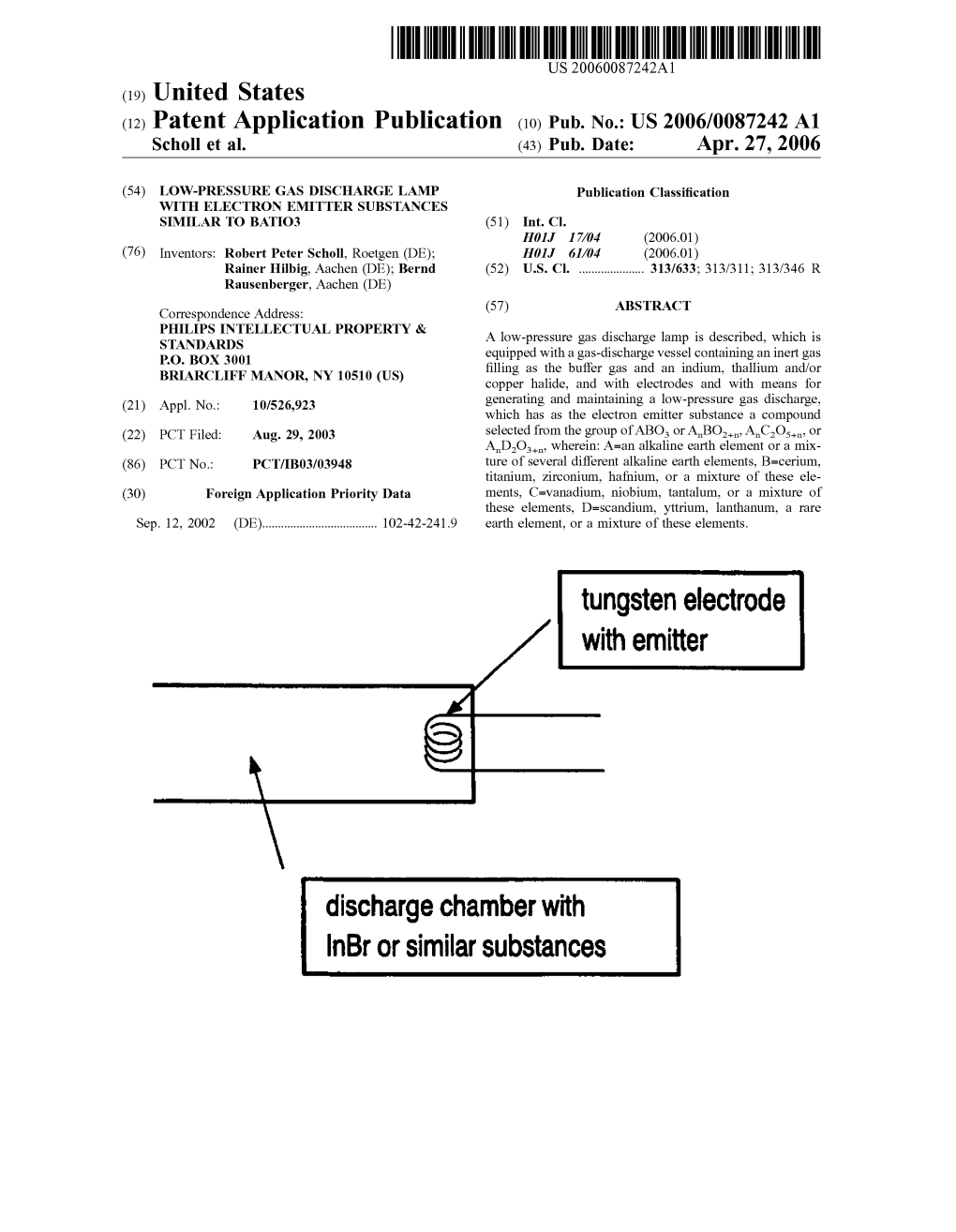
Tungsten Electrode with Emitter
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Buffer Gas Experiments in Mercury (Hg+) Ion Clock
Buffer Gas Experiments in Mercury (Hg+) Ion Clock Sang K. Chung, John D. Prestage, Robert L. Tjoelker, Lute Maleki California Institute of Technology, Jet Propulsion Laboratory 4800 Oak Grove Drive, Bldg 298 Pasadena, CA 91109 Abstract—We report measured buffer gas collision shifts of the environment and stable helium pressure this system is not 40.507347996xx GHz mercury ion clock transition using inert sufficient to meet mass, power, size, and operability helium, neon, and argon gases and getterable molecular considerations of a space clock [6]. hydrogen and nitrogen gases. The frequency shift due to Two buffer gas / vacuum pump combination are currently methane was also examined. At low partial pressures methane being considered for possible operation of a small space-born gas did not impact the trapped ion number but was observed to strongly relax the population difference in the two hyperfine clock. One approach uses a small getter pump with a fixed clock states thereby reducing the clock resonance signal. A amount of inert buffer gas. Two issues that must be similar population relaxation was also observed for other understood are the long term buffer gas pressure stability and molecular buffer gases (N2, H2) but at much reduced rate. possible evolution of non-getterable gas that could potentially adversely affect clock stability or operation. A second approach is to use a miniature ion pump and a calibrated I. INTRODUCTION micro-leak buffer gas that is not inert (inert gas can shorten In recent years, mercury ion (199Hg+) clock technology has ion pump life very quickly). demonstrated excellent inherent stability, reaching ~3 x 10-16 long-term frequency stability with continuous, free running II. -

Development and Applications of Proton Transfer Reaction-Mass Spectrometry for Homeland Security: Trace Detection of Explosives
DEVELOPMENT AND APPLICATIONS OF PROTON TRANSFER REACTION-MASS SPECTROMETRY FOR HOMELAND SECURITY: TRACE DETECTION OF EXPLOSIVES by Ramón González-Méndez, MSc A thesis submitted to the University of Birmingham for the degree of DOCTOR OF PHILOSOPHY School of Physics and Astronomy The University of Birmingham, UK February 2017 University of Birmingham Research Archive e-theses repository This unpublished thesis/dissertation is copyright of the author and/or third parties. The intellectual property rights of the author or third parties in respect of this work are as defined by The Copyright Designs and Patents Act 1988 or as modified by any successor legislation. Any use made of information contained in this thesis/dissertation must be in accordance with that legislation and must be properly acknowledged. Further distribution or reproduction in any format is prohibited without the permission of the copyright holder. Abstract This thesis investigates the challenging task of sensitive and selective trace detection of explosive compounds by means of proton transfer reaction mass spectrometry (PTR-MS). In order to address this, new analytical strategies and hardware improvements, leading to new methodologies and analytical tools, have been developed and tested. These are, in order of the Chapters presented in this thesis, the switching of reagent ions, the implementation of a novel thermal desorption unit, and the use of an ion funnel drift tube or fast reduced electric field switching to modify the ion chemistry. In addition to these, a more fundamental study has been undertaken to investigate the reactions of picric acid (PiA) with a number of different reagent ions. The novel approaches described in this thesis have improved the PTR-MS technique by making it more versatile in terms of its analytical performance, namely providing assignment of chemical compounds with high confidence. -

Enhanced Escape Rate for Hg 254Nm Resonance Radiation in Fluorescent Lamps
Home Search Collections Journals About Contact us My IOPscience Enhanced escape rate for Hg 254 nm resonance radiation in fluorescent lamps This content has been downloaded from IOPscience. Please scroll down to see the full text. 2013 J. Phys. D: Appl. Phys. 46 415204 (http://iopscience.iop.org/0022-3727/46/41/415204) View the table of contents for this issue, or go to the journal homepage for more Download details: IP Address: 128.104.46.196 This content was downloaded on 24/09/2013 at 02:20 Please note that terms and conditions apply. IOP PUBLISHING JOURNAL OF PHYSICS D: APPLIED PHYSICS J. Phys. D: Appl. Phys. 46 (2013) 415204 (8pp) doi:10.1088/0022-3727/46/41/415204 Enhanced escape rate for Hg 254 nm resonance radiation in fluorescent lamps James E Lawler1 and Mark G Raizen2 1 Department of Physics, University of Wisconsin, Madison, WI 53706, USA 2 Center for Nonlinear Dynamics and Department of Physics, University of Texas, Austin, TX 78712, USA Received 7 June 2013, in final form 7 August 2013 Published 23 September 2013 Online at stacks.iop.org/JPhysD/46/415204 Abstract The potential of the low-cost MAGIS isotopic separation method to improve fluorescent lamp efficacy is explored using resonance radiation transport simulations. New Hg isotopic mixes are discovered that yield escape rates for 254 nm Hg I resonance radiation equal to 117% to 122% of the rate for a natural isotopic mix under the same lamp conditions. (Some figures may appear in colour only in the online journal) 1. Introduction will represent over 75% of all lighting sales by 2030, resulting in an annual primary energy savings of 3.4 quads.’ Fluorescent lighting is the technology of choice for almost Even after inorganic LED and/or organic LED (OLED) all non-residential indoor lighting applications around the technology achieve competitive performance and cost levels, world. -

Energy Transfer Processes in Electron Beam Excited Mixtures of Laser Dye Vapors with Rare Gases G
Energy transfer processes in electron beam excited mixtures of laser dye vapors with rare gases G. Marowsky Max-Planck Institut fur Biophysikalische Chemie. D 3400 Gottingen. Federal Republic of Germany R. Cordray, F. Tittel, and B. Wilson Electrical Engineering Department, Rice University. Houston. Texas 77001 J. W. Keto Physics Department. University of Texas at Austin. Austin. Texas 78712 (Received 26 May 1977) The energy transfer mechanisms for electron beam excited N -92 dye vapor in various bU!fer gas mixtur.es have been examined. Optimum conditions for achieving intense dye vapor fluorescence with short duratIOn electron pulse excitation are reported. INTRODUCTION colliSional excitation from the metastable states of the Direct electrical excitation of dye vapor lasers has rare gas buffer atoms and molecules and electron im pact excitation appear to be the dominant processes for received considerable attention in recent years. l,Z Elec trical excitation offers the possibilities of overcoming coupling electron beam energy to the dye vapor. By op timizing the buffer gas composition, the total pressure, the inherent limitations in efficiency for optically pumped dye lasers, extending the present uv wavelength limit and the electron beam deposition it is possible to en hance the fluorescence yields of excited state populations of about 320 nm to shorter wavelengths, and scaling to of buffer gas-dye vapor mixtures. Due to the complex high power laser systems. In this paper we report re ity of these mixtures under high levels of eXCitation, no sults of a study to identify the optimum energy transfer quantitative measurements on kinetic parameters such conditions which produce efficient fluorescence yields as relevant rate constants and colliSional and radiative for electron beam excited high pressure buffer gas-dye lifetimes have been obtained as yet. -

APPLICATION NOTE LD20-05 Multidetek2 Gas Chromatograph with Plasmadetek2 & TCD Detectors Uses for the Analysis of Purity Xenon-Krypton-Neon
APPLICATION NOTE LD20-05 MultiDetek2 gas chromatograph with PlasmaDetek2 & TCD detectors uses for the analysis of purity Xenon-Krypton-Neon MultiDetek2 PlasmaDetek2 The noble gases also called inert gases or rare gases have several characteristics that make them important and unique as: low reactivity, low thermal conductivity and high stability, among others. Being at very low concentration in the earth’s atmosphere, it makes these gases very expensive to produce. The six naturally noble gases are Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe) and the radioactive Radon (Rn). The rarest gases of these are Xenon, Krypton and Neon making them very expensive to use for industrial applications. NEON/KRYPTON/XENON MAJOR APPLICATIONS: Aerospace: Xenon is used for the following aerospace applications: satellite programs, space travel, propulsion agent for spacecraft, satellite thruster and interplanetary probe. Electronics: These rare gases, can be used in many electronics applications such as: excimer lasers, buffer gas used in lasers for semiconductor manufacturing, deep trench etching of DRAM integrated circuits, focused etch process, and plasma panel display. Glass: Krypton is used as a filler in the production of double and triple-pane insulated windows. Major advantages of using krypton are reducing heat loss, increasing heat transfer resistance in the unit, and reducing levels of solar radiation. You can also increase the R-value or decrease the U-factor for window and door insulation with krypton, xenon and rare gas mixtures. Lasers: Neon-based excimer lasers are utilized for etching silicon wafers, LASIK eye surgery, micro-machining organic materials, UV lithography in integrated circuit fabrication, micro drilling, He/Ne mixes for optical readers, and wafer dicing. -

"The Buffer-Gas Positron Accumulator and Resonances in Positron
Written in conjunction with the symposium honoring the retirement of Dick Drachman and Aaron Temkin (NASA Goddard Space Flight Center, Greenbelt, MD; November 18, 2005); to be published by the NASA Conference Publication Service, A. Bhatia, ed. (2006). THE BUFFER-GAS POSITRON ACCUMULATOR AND RESONANCES IN POSITRON-MOLECULE INTERACTIONS C. M. Surko Department of Physics, University of California, San Diego, La Jolla CA 92093 ABSTRACT This is a personal account of the development of our buffer-gas positron trap and the new generation of cold beams that these traps enabled. Dick Drachman provided much appreciated advice to us from the time we started the project. The physics underlying trap operation is related to resonances (or apparent resonances) in positron- molecule interactions. Amusingly, experiments enabled by the trap allowed us to understand these processes. The positron-resonance “box score” to date is one resounding “yes,” namely vibrational Feshbach resonances in positron annihilation on hydrocarbons; a “probably” for positron-impact electronic excitation of CO and N2; and a “maybe” for vibrational excitation of selected molecules. Two of these processes enabled the efficient operation of the trap, and one almost killed it in infancy. We conclude with a brief overview of further applications of the trapping technology discussed here, such as “massive” positron storage and beams with meV energy resolution. IT ALL STARTED AT LUNCH WITH MARV Since this paper is written in conjunction with the symposium marking the retirement of Dick Drachman and Aaron Temkin, I depart from third-person style to relate a personal view of the development of the buffer-gas trap and the physics that has come from it. -

Laser Cooling of Dense Rubidium-Noble Gas Mixtures Via Collisional Redistribution of Radiation
Laser Cooling of Dense Rubidium-Noble Gas Mixtures via Collisional Redistribution of Radiation Ulrich Vogla,†, Anne Saßa and Martin Weitza a Institut f¨ur Angewandte Physik der Universit¨at Bonn, Wegelerstr. 8, 53115 Bonn, Germany ABSTRACT We describe experiments on the laser cooling of both helium-rubidium and argon-rubidium gas mixtures by collisional redistribution of radiation. Frequent alkali-noble gas collisions in the ultradense gas, with typically 200 bar of noble buffer gas pressure, shift a highly red detuned optical beam into resonance with a rubidium D- line transition, while spontaneous decay occurs close to the unshifted atomic resonance frequency. The technique allows for the laser cooling of macroscopic ensembles of gas atoms. The use of helium as a buffer gas leads to smaller temperature changes within the gas volume due to the high thermal conductivity of this buffer gas, as compared to the heavier argon noble gas, while the heat transfer within the cell is improved. Keywords: Laser cooling, optical refrigeration, collisional redistribution 1. INTRODUCTION The idea to cool matter with light can be traced back at least 80 years,1 but not until the invention of the laser viable proposals and experimental work has started. The greatest influence on further research in this area had the technique of Doppler cooling of atomic gases suggested by H¨ansch and Schawlow,2 which has lead to the extremely successful field of ultracold dilute atomic gases.3–5 In another approach for laser cooling, anti-Stokes processes in multilevel systems have been employed. Proof of principle experiments for this type of laser cooling 6, 7 8 have been carried out with CO2 gas and a dye-solution. -

Krypton Isotope Analysis Using Near-Resonant Stimulated Raman Spectroscopy
PNL-10203 UC-713 Krypton Isotope Analysis Using Near-Resonant Stimulated Raman Spectroscopy C. A. Whitehead B. D. Cannon J. F. Wacker December 1994 Prepared for the U.S. Department of Energy under Contract DE-AC06-76RLO 1830 Pacific Northwest Laboratory Richland, Washington 99352 MASTER D.STRIBUTION OF TH.S DOCUMENT IS UNUNITED f DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, make any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. DISCLAIMER Portions of this document may be illegible in electronic image products. Images are produced from the best available original document. EXECUTIVE SUMMARY The Krypton Isotope Laser Analysis (KILA) method has been developed at the Pacific Northwest Laboratory in an attempt to measure small (typically, <10"10) ratios of unstable to. stable krypton isotopes in air samples. This technique uses high-resolution lasers to induce a specific isotope of krypton to fluoresce for optical detections. -

PTR-MS in Italy: a Multipurpose Sensor with Applications in Environmental, Agri-Food and Health Science
Sensors 2013, 13, 11923-11955; doi:10.3390/s130911923 OPEN ACCESS sensors ISSN 1424-8220 www.mdpi.com/journal/sensors Review PTR-MS in Italy: A Multipurpose Sensor with Applications in Environmental, Agri-Food and Health Science Luca Cappellin 1, Francesco Loreto 2,*, Eugenio Aprea 1, Andrea Romano 1, José Sánchez del Pulgar 1, Flavia Gasperi 1 and Franco Biasioli 1 1 Research and Innovation Centre, Fondazione Edmund Mach (FEM), Via E. Mach 1, San Michele all’Adige 38010, Italy; E-Mails: [email protected] (L.C.); [email protected] (E.A.); [email protected] (A.R.); [email protected] (J.S.P.); [email protected] (F.G.); [email protected] (F.B.) 2 Institute of Agro-Environmental and Forest Biology, National Research Council, Via Salaria km 29, 300-00015 Monterotondo Scalo, Roma, Italy * Author to whom correspondence should be addressed; E-Mail: [email protected]. Received: 5 July 2013; in revised form: 23 August 2013 / Accepted: 27 August 2013 / Published: 9 September 2013 Abstract: Proton Transfer Reaction Mass Spectrometry (PTR-MS) has evolved in the last decade as a fast and high sensitivity sensor for the real-time monitoring of volatile compounds. Its applications range from environmental sciences to medical sciences, from food technology to bioprocess monitoring. Italian scientists and institutions participated from the very beginning in fundamental and applied research aiming at exploiting the potentialities of this technique and providing relevant methodological advances and new fundamental indications. In this review we describe this activity on the basis of the available literature. -

Ionization Processes in Fluorescent Lamps:… Physical Review E 71, 056404 ͑2005͒
PHYSICAL REVIEW E 71, 056404 ͑2005͒ Ionization processes in fluorescent lamps: Evaluation of the Hg chemi-ionization rate coefficients Valery A. Sheverev,1 Graeme G. Lister,2 and Vadim Stepaniuk1 1Polytechnic University, Six Metrotech Center, Brooklyn, New York 11201, USA 2OSRAM SYLVANIA, 71 Cherry Hill Drive, Beverly, Massachusetts 01915, USA ͑Received 23 March 2004; revised manuscript received 30 November 2004; published 13 May 2005͒ Chemi-ionization by two mercury atoms to produce mercury atomic and molecular ions has been considered an important process in fluorescent lamps ͑FLs͒ for a quarter of a century. Despite the absence of reliable data, these processes have been included in a number of numerical models to help explain some of the experimental observations. These models have shown that the most important process is the Penning ionization of two Hg ͑ 3 ͒ ͑ 3 ͒! + ͑ 1 ͒ metastable atoms Hg 6 P2 +Hg 6 P2 Hg +Hg 6 S0 +e. Although there is no experimental measurement of this cross section, modelers have typically used values implied from measurements of other chemi- ionization cross sections, or values obtained by fitting parameters to numerical models to obtain agreement with experiment. Recent theoretical investigations have indicated that the cross sections for the important processes may not be as large as previously thought. The aim of the present paper is to critically review the historical development of studies of chemi-ionization in fluorescent lamps and to present new experimental evidence which is consistent with the theoretical calculations and contradicts the conclusions from previously published experiments. DOI: 10.1103/PhysRevE.71.056404 PACS number͑s͒: 52.20.Hv, 52.25.Jm I. -

The Analysis of Modern Low Pressure Amalgam Lamp Characteristics
The analysis of modern low pressure amalgam lamp characteristics Michiel Van der Meer (Philips Lighting), Fred van Lierop (LSI/Lighttech), Dmitry Sokolov (LIT) Over the last 10-15 years the market of manufacturers of UV equipment for water, air and surface disinfection, as well as the technology of UV disinfection has made a significant progress. It is valid to say that the market of UV equipment manufactures has shaped with the main players being Trojan (Canada), Wedeco Xylem (USA- Germany), LIT Technology (Russia/Germany), Calgon Carbon (USA), HALMA Fluid Technology Group and many others. The rapid growth of UV technology is to a large extent caused by successful development of the irradiation source technology at a wavelength of 254 nm – a low pressure amalgam lamp. We can remember that even 10 years ago the main source of irradiation was lamps with power up to 100 W generally made of so called soft glass. There were also similar mercury lamps made of quartz glass, which we know as standard low pressure mercury lamps. The developing technology has brought to live new sources with powers of 200, 300, 500 and even 900 W. They made it possible to significantly reduce capital expenses by minimizing costs of UV systems which are now equipped with less number of lamp-units but maintain the same level of disinfection and water flow. The largest producers of modern low pressure amalgam lamps are Heraeus Noblelight (Germany), LSI/Lighttech (USA/Hungary), Philips Lighting (Belgum/China), LIT (Russia), Wedeco Xylem (Germany), UV- Technik/Hoenle group (Germany), First Light (USA) and some others. -

Absorption Spectroscopy of Xenon and Ethylene-Noble Gas Mixtures at High Pressure: Towards Bose-Einstein Condensation of Vacuum Ultraviolet Photons
Absorption spectroscopy of xenon and ethylene-noble gas mixtures at high pressure: Towards Bose-Einstein condensation of vacuum ultraviolet photons Christian Wahl, Rudolf Brausemann, Julian Schmitt, Frank Vewinger, Stavros Christopoulos, Martin Weitz∗ Institut f¨urAngewandte Physik, Universit¨atBonn Wegelerstraße 8, 53115 Bonn, Germany Bose-Einstein condensation is a phenomenon well known for material particles as cold atomic gases, and this concept has in recent years been extended to photons confined in microscopic optical cavities. Essential for the operation of such a photon condensate is a thermalization mechanism that conserves the average particle number, as in the visible spec- tral regime can be realized by subsequent absorption re-emission processes in dye molecules. Here we report on the status of an experimental effort aiming at the extension of the concept of Bose-Einstein condensation of photons towards the vacuum ultraviolet spectral regime, with gases at high pressure conditions serving as a thermalization medium for the photon gas. We have recorded absorption spectra of xenon gas at up to 30 bar gas pressure of the 5p6 − 5p56s transition with a wavelength close to 147 nm. Moreover, spectra of ethylene noble gas mixtures between 155 nm and 180 nm wavelength are reported. I. INTRODUCTION For bosonic particles with a non-vanishing rest mass Bose-Einstein condensation to a macroscopi- cally occupied ground state minimizes the free energy when cooled to very low temperature given a sufficient particle density [1{4]. In distinct contrast, Bose-Einstein condensation usually does not occur for photons [5]. For the perhaps most widely discussed photon gas, blackbody radiation [6, 7], photons disappear in the system walls when cooled to low temperature instead of exhibiting condensation to the ground mode.