CTRI Trial Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Payment Locations - Muthoot
Payment Locations - Muthoot District Region Br.Code Branch Name Branch Address Branch Town Name Postel Code Branch Contact Number Royale Arcade Building, Kochalummoodu, ALLEPPEY KOZHENCHERY 4365 Kochalummoodu Mavelikkara 690570 +91-479-2358277 Kallimel P.O, Mavelikkara, Alappuzha District S. Devi building, kizhakkenada, puliyoor p.o, ALLEPPEY THIRUVALLA 4180 PULIYOOR chenganur, alappuzha dist, pin – 689510, CHENGANUR 689510 0479-2464433 kerala Kizhakkethalekal Building, Opp.Malankkara CHENGANNUR - ALLEPPEY THIRUVALLA 3777 Catholic Church, Mc Road,Chengannur, CHENGANNUR - HOSPITAL ROAD 689121 0479-2457077 HOSPITAL ROAD Alleppey Dist, Pin Code - 689121 Muthoot Finance Ltd, Akeril Puthenparambil ALLEPPEY THIRUVALLA 2672 MELPADAM MELPADAM 689627 479-2318545 Building ;Melpadam;Pincode- 689627 Kochumadam Building,Near Ksrtc Bus Stand, ALLEPPEY THIRUVALLA 2219 MAVELIKARA KSRTC MAVELIKARA KSRTC 689101 0469-2342656 Mavelikara-6890101 Thattarethu Buldg,Karakkad P.O,Chengannur, ALLEPPEY THIRUVALLA 1837 KARAKKAD KARAKKAD 689504 0479-2422687 Pin-689504 Kalluvilayil Bulg, Ennakkad P.O Alleppy,Pin- ALLEPPEY THIRUVALLA 1481 ENNAKKAD ENNAKKAD 689624 0479-2466886 689624 Himagiri Complex,Kallumala,Thekke Junction, ALLEPPEY THIRUVALLA 1228 KALLUMALA KALLUMALA 690101 0479-2344449 Mavelikkara-690101 CHERUKOLE Anugraha Complex, Near Subhananda ALLEPPEY THIRUVALLA 846 CHERUKOLE MAVELIKARA 690104 04793295897 MAVELIKARA Ashramam, Cherukole,Mavelikara, 690104 Oondamparampil O V Chacko Memorial ALLEPPEY THIRUVALLA 668 THIRUVANVANDOOR THIRUVANVANDOOR 689109 0479-2429349 -

List of Teachers Posted from the Following Schools to Various Examination Centers As Assistant Superintendents for Higher Secondary Exam March 2015
LIST OF TEACHERS POSTED FROM THE FOLLOWING SCHOOLS TO VARIOUS EXAMINATION CENTERS AS ASSISTANT SUPERINTENDENTS FOR HIGHER SECONDARY EXAM MARCH 2015 08001 - GOVT SMT HSS,CHELAKKARA,THRISSUR 1 DILEEP KUMAR P V 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR 04884231495, 9495222963 2 SWAPNA P 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR , 9846374117 3 SHAHINA.K 08035-GOVT. RSR VHSS, VELUR, THRISSUR 04885241085, 9447751409 4 SEENA M 08041-GOVT HSS,PAZHAYANNOOR,THRISSUR 04884254389, 9447674312 5 SEENA P.R 08046-AKM HSS,POOCHATTY,THRISSUR 04872356188, 9947088692 6 BINDHU C 08062-ST ANTONY S HSS,PUDUKAD,THRISSUR 04842331819, 9961991555 7 SINDHU K 08137-GOVT. MODEL HSS FOR GIRLS, THRISSUR TOWN, , 9037873800 THRISSUR 8 SREEDEVI.S 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR , 9020409594 9 RADHIKA.R 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR 04742552608, 9847122431 10 VINOD P 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR , 9446146634 11 LATHIKADEVI L A 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR 04742482838, 9048923857 12 REJEESH KUMAR.V 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR 04762831245, 9447986101 08002 - GOVT HSS,CHERPU,THRISSUR 1 PREETHY M K 08003-GOVT MODEL GHSS, IRINJALAKKUDA, THRISSUR 04802820505, 9496288495 2 RADHIKA C S 08003-GOVT MODEL GHSS, IRINJALAKKUDA, THRISSUR , 9495853650 3 THRESSIA A.O 08005-GOVT HSS,KODAKARA,THRISSUR 04802726280, 9048784499 4 SMITHA M.K 08046-AKM HSS,POOCHATTY,THRISSUR 04872317979, 8547619054 5 RADHA M.R 08050-ST ANTONY S HSS,AMMADAM,THRISSUR 04872342425, 9497180518 6 JANITHA K 08050-ST ANTONY S HSS,AMMADAM,THRISSUR 04872448686, 9744670871 1 7 SREELEKHA.E.S 08050-ST ANTONY S HSS,AMMADAM,THRISSUR 04872343515, 9446541276 8 APINDAS T T 08095-ST. PAULS CONVENT EHSS KURIACHIRA, THRISSUR, 04872342644, 9446627146 680006 9 M.JAMILA BEEVI 08107-SN GHSS, KANIMANGALAM, THRISSUR, 680027 , 9388553667 10 MANJULA V R 08118-TECHNICAL HSS, VARADIAM, THRISSUR, 680547 04872216227, 9446417919 11 BETSY C V 08138-GOVT. -

Location Accessibility Contact
Panchayath/ Municipality/ Koratty Panchayat Corporation LOCATION District Thrissur Nearest Town/ Koratty – 550 m Landmark/ Junction Nearest Bus statio Koratty Junction Bus Stop – 450 m Nearest Railway Irinjalakuda Railway Station – 13.8 km statio ACCESSIBILITY Nearest Airport Cochin International Airport – 17 km St. Mary's Forane Church, Koratty, Thrissur, Kerala, India, 680308 Ph : 0480 2732780, 2734009 Email : [email protected] CONTACT Website: www.korattymuthy.com DATES FREQUENCY DURATION TIME October Annually 19 days ABOUT THE FESTIVAL (Legend/History/Myth) The church has many popular beliefs woven around it. Six centuries back Kerala was divided into numerous provinces ruled by kings and landlords. Two of such neighbouring landlords were Koratty Kaimal and Kodassery Kartha. The descendents of these two feudal lords live in Koratty even now, and this adds on to the authenticity of this belief about the origin of the Koratty Church. Under the feudal lord, Koratty Kaimal, many Christians served in the army. In a battle between Kaimal and Kartha many were killed. Sri Kavalakkadan Kochu Vareed, the commander of the Kaimal army was one among the dead. At the time the Catholics had only one church in that area - at Ambazhakkad. Kaimal made all arrangements for the funeral of Sri. Kochu Vareed to be held in full military honours and religious rituals at the Ambazhakkad church. But his rival, Kodassery Kartha interfered and the burial could not take place there. The funeral procession had to return. In between, the pall-bearers had to rest and they placed the coffin down. When they tried to lift the coffin again they found to their astonishment, that it was firmly stuck to the ground and they could not lift. -

Service Electoral Roll - 2017
SERVICE ELECTORAL ROLL - 2017 DIST_NO & NAME: 7 Thrissur AC_NO & NAME:- 72 72-CHALAKKUDY ECI CODE NAME SEX TYPE HOUSE ADDRESS REGIMENTAL ADDRESS 106577 VARGHESE JOY T M M THOTTATHIL Army Arty Records MURINGOOR THEKKK MUKUNDHA PUR Arty Records, PIN - 908 802, c/o 56 APO Nalukettu 1250163 SANDEEP KC M M KISHAKKE Army PANANJIKUNNATH Armd Corps Records KODAKARA KODAKARA Armd Corps Records, PIN - 900 476, c/o 56 A PERAMBRA 680689 1302376 K UNNIKRISHNAN M M 2/338 INDIAN AIR FORCE DPS KODAKARA CHALAKUDY DPS AIR HQ NEW DELHI KODAKARA 1292329 SYAM KS M M 453 A KEEZHARA HOU INDIAN AIR FORCE MELOOR DPS MELOOR MUKUNDAPURA DPS AIR HQ NEW DELHI MELOOR 1292941 PRAJITH MURALI A M M INDIAN AIR FORCE NEAR PANAMPILLY CO DPS CHALAKUDY MUKUNDAPURA DPS AIR HQ NEW DELHI POTTA 1337464 KM VIJAYAN M M 96A INDIAN AIR FORCE POOLANI DPS MELOOR CHALAKUDY DPS AIR HQ NEW DELHI MELOOR 1337701 K V SURENDHRAN M M KOOTTUNGAL INDIAN AIR FORCE THAIKKOOTTAM DPS KALLOOR VADAKKUM CHALAKUDI DPS AIR HQ NEW DELHI PALAYAMPARAMBU 35557 SURYA GOVIND MV M M Army Arty Records MELOOR MUKUNDAPURA Arty Records, PIN - 908 802, c/o 56 APO KUNNAPILLY 680311 67812 MANU CHANDRAN M M POTHIKARA Army Arty Records KODAKARA CHALAKUDY Arty Records, PIN - 908 802, c/o 56 APO MANAKULANGARA 680684 69197 JOBY M V M M Army Arty Records Pariyaram Mukundrapuram Arty Records, PIN - 908 802, c/o 56 APO Vettilappara 49143 SHAJU JOSE M M Army Arty Records Muringoor Thekkumm Mukundapuram Arty Records, PIN - 908 802, c/o 56 APO Koratty 1300275 BINEESH KUMAR K K M M INDIAN AIR FORCE PARIYARAM DPS PARIYARAM CHALAKKUDY -

Accused Persons Arrested in Thrissur Rural District from 01.02.2015 to 07.01.2015
Accused Persons arrested in Thrissur Rural district from 01.02.2015 to 07.01.2015 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Cr. No & Sec Police father of Address of Accused which Time of Officer, Rank which No. Accused Sex of Law Station Accused Arrested Arrest & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 C.J CR 123/15 KATTISSERY MUHAMMED U/S 118(i) HOUSE, 01.02.201 LATHEEF , JFCM MOHANAN. 48/15 PUNNUKKA KP ACT & VADAKKEK 1 KUNJUMON MUKKUTHALA, 5 AT 1800 SI OF KUNNAMKU D MALE VU 6(b) r/w 24 AD NANNAMMUKKU, HRS POLICE LAM OF COTPA MALAPPURAM VADAKKEKA ACT D PS K.VISWANAT MELEMBAT KUNNATHUPEEDI 01.02.201 CR 78/15 HAN, SI OF JFCM 49/15 TUMUKKU, WADAKKA 2 SIDHIK BAPPUTTY KAYIL HOUSE, 5 AT 1800 U/S 15 OF POLICE WADAKKAN MALE KUMARANE NCHERY MELEMBATTU HRS KG ACT WADAKKAN CHERY LLUR CHERY PS K.VISWANAT VAKAPARAKKAN MELEMBAT 01.02.201 CR 78/15 HAN, SI OF JFCM SREENIVA KRISHNANK 35/15 NEAR TUMUKKU, WADAKKA 3 5 AT 1800 U/S 15 OF POLICE WADAKKAN SAN UTTY MALE VAZHAMKODE KUMARANE NCHERY HRS KG ACT WADAKKAN CHERY MUSLIM PALLY LLUR CHERY PS K.VISWANAT CHAZHIYOTTIL MELEMBAT 01.02.201 CR 78/15 HAN, SI OF JFCM 32/15 HOUSE, TUMUKKU, WADAKKA 4 RAJESH SANKUNNY 5 AT 1800 U/S 15 OF POLICE WADAKKAN MALE NATTYANCHIRA, KUMARANE NCHERY HRS KG ACT WADAKKAN CHERY CHELAKKARA LLUR CHERY PS K.VISWANAT KOOLAYIL MELEMBAT 01.02.201 CR 78/15 HAN, SI OF JFCM BHASKARA 44/15 HOUSE, TUMUKKU, WADAKKA 5 ACHOOTTY 5 AT 1800 U/S 15 OF POLICE WADAKKAN N MALE KODUMBU KUMARANE NCHERY HRS KG ACT WADAKKAN CHERY CHATHANCHIRA LLUR CHERY PS K.VISWANAT MELEMBAT CHEMBATHETHIL 01.02.201 CR 78/15 HAN, SI OF JFCM 22/15 TUMUKKU, WADAKKA 6 SHEREEF LATHEEF HOUSE, 5 AT 1800 U/S 15 OF POLICE WADAKKAN MALE KUMARANE NCHERY CHATHAN CHIRA HRS KG ACT WADAKKAN CHERY LLUR CHERY PS CR 71/15 U/S 379 r/w 114 IPC & 20 OF V.M. -

Joseph Cheeran / Cheeranachen
Joseph Cheeran / Cheeranachen Rev. Fr. Dr. Joseph Cheeran – popularly called ‘Cheeranachen’ is a renowned church historian, orator, writer and a scholar of liturgy of Malankara Orthodox Church , India and a Malayalam language retired professor from Union Christian College, Alwaye . He is a priest of Indian Orthodox church and have 40 publication of books in Malayalam language and still contributing his research works in the history of Malankara/Indian orthodox Syrian Christians . He has published more than 250 research articles in various church publications like The Church Weekly, Malankara Sahba , Kunjadukalude Velicham, Bhashaposhini, Edavaka pathrika, etc. He was a resource person for State Institute of Education, Trivandrum. He conducted several classes and talks through All India Radio, Trichur in 1970s and 1980s. Fr.Joseph Cheeran was born in Trichur District in Pazhanji Village as the grandson of Rev.Fr.Geevarghese Cheeran on 17th August in 1945. Name of his parents were Mathappan and Kunjathiry. His mother died when he was three years old. However, he achieved First Rank from University of Kerala for Malayalam Vidwan Examination. He achieved Ph.D degree from Mahatma Gandhi University, Kottayam for the topic 'Influence of Bible on Modern Malayalam Poetry'. He worked as a school teacher at CMS High School, Trichur before he joined as faculty of Malayalam in U.C.College, Alwaye. He achieved his Post Graduation Degree in Malayalam as private candidate. In school and college certificates, his name is written as C.M.Jose. Liturgical Guru of Fr. Joseph Cheeran is the famous sound scholar H.G.Yuhanon Mar Severiose . Priestly ordinations of different stages were from his Malpan – Guru, Severiose Thirumeni. -

Brief Industrial Profile of Thrissur District
1 Government of India Ministry of MSME Brief Industrial Profile of Thrissur District Carried out by MSME-Development Institute (Ministry of MSME, Govt. of India,), Thrissur Phone-0487-2360536, 2360638 Fax: 0487-2360216 e-mail: [email protected] Web-www.msmedithrissur.gov.in 2 Contents S. No. Topic Page No. 1. General Characteristics of the District 1 1.1 Location & Geographical Area 1 1.2 Topography 1 1.3 Availability of Minerals. 2 1.4 Forest 2 1.5 Administrative set up 3 2. District at a glance 3,4,5 2.1 Existing Status of Industrial Area in Thrissur District. 5 3. Industrial Scenario Of Thrissur District. 6 3.1 Industry at a Glance 6 3.2 Year Wise Trend Of Units Registered 7 3.3 Details Of Existing Micro & Small Enterprises & Artisan Units In The 8 District 3.4 Large Scale Industries / Public Sector undertakings 9 3.5 Major Exportable Item 9 3.6 Growth Trend 9 3.7 Vendorisation / Ancillarisation of the Industry 10 3.8 Medium Scale Enterprises 10 3.8.1 List of the units in Thrissur & near by Area 10 3.8.2 Major Exportable Item 10 3.9 Service Enterprises 10 3.9.1 Coaching Industry 10 3.9.2 Potentials areas for service industry 10 3.10 Potential for new MSMEs 10 4. Existing Clusters of Micro & Small Enterprise 10,11 4.1 Detail Of Major Clusters 11 4.1.1 Manufacturing Sector 11 4.1.2 Service Sector 11 4.2 Details of Identified cluster 11 4.2.1 -do- 11 4.2.2 -do- 11 4.2.3 -do- 12 4.2.4 -do- 12 4.2. -

Jananeethi Report 1 NGIL Pollution Facts Finding Team from Jananeethi Institute
Jananeethi Report 1 NGIL Pollution Facts Finding Team from Jananeethi Institute 1. Advocate George Pulikuthiyil, M.A, LL B, B.Th, B.Ph. Executive Director, Jananeethi & Jananeethi Institute, Thrissur, Kerala, India. 2. Dr.Gigi Joseph, Ph D Lecturer, Dept. of Zoology, St.Thomas College, Ranni, Kerala 3. Dr.George Mathen, MV Sc Professor (retd), Animal Nutrition; Veterinary College , KAU & Jananeethi, Kerala, India 4. Er. Paul Joseph Kattookaren FIE, Chartered Engineer & Surveyor, Jananeethi, Kerala, India. 5. Prof. N.N.Gokuldas, M Sc Professor (retd), Dept. of Biology, Sree Krishna College, Guruvayoor, Kerala, India. 6. Advocate P.Pramod, LL M District Court, Thrissur; Member, Director Board, Jananeethi, Kerala, India. 7. Advocate P.Sunilkumar, LL M Law Officer, Jananeethi Institute, Thrissur, Kerala, India 8. Mr. Ajayakumar Melveetil, B.A. Photography & Documentation Technical Advice : 1. Dr.Francis Xavier, Ph D Professor & Head, CBF, Thumburmuzhy; KAU, Kerala; Jananeethi Institute & Member IUCN, SSC 2. Dr.Davaki Girija, Ph D Professor, Centre for Plant Bio-technology & Molecular Biology; KAU, Kerala, India. Scientific Analysis of samples conducted by: Dr.P.Sureshkumar, Ph D Professor & Principal Investigator, RTL, KAU, Kerala, India. At the Radiotracer Laboratory, College of Horticulture, Kerala Agricultural University, Thrissur, Kerala 680656, India. Technical assistance by Mr.Suresh Babu, Jananeethi Institute, Thrissur Ms.Bhadra R.Menon, Christ Law School, Bangalore Mr.Balu M.Nair, National University of Juridical Sciences, W.B. For Correspondence JANANEETHI INSTITUTE, P.B. No. 8, Mannuthy P.O., Thrissur – 680651, Kerala, India. Tel: 91-487-2373479; Fax: 91-487-2373281, Email: [email protected]; [email protected] Web : http://www.jananeethi.org Acknowledgement This study was under taken and the report is being published in public interest only. -

House Details Chalakudy Damage Type Complete Loss of Buildings
House Details_Chalakudy_Damage Type_Complete loss Of Buildings Ward House Sub Sl No Localbody Type Localbody Name Taluk Name Village Name Owner Name Owner Address Damage Type No No No 1 Grama Panchayat Alur 1 17 Chalakudy Kallettumkara sajeevan kocheri house,panjappilly,p.o kallettunkara Complete loss of Buildings Chakkedath(H) vellanchira po vellanchira, 2 Grama Panchayat Alur 9 187 Chalakudy Aloor Jaison chakko Complete loss of Buildings alur, Thrissur 680697 ILLIKKAL,THURUTHIPARAMBU 3 Grama Panchayat Alur 9 384 Chalakudy Aloor Rosy Antony VELLANCHIRA,ANNALLUR P Complete loss of Buildings O,Annallur,Thrissur,Kerala,680731 Nambiaruveettil House,Annallur P 4 Grama Panchayat Alur 10 132 Chalakudy Aloor N K VWLAYUDHAN O,Thiruthiparambu,Annallur,Thrissur,Kerala Complete loss of Buildings ,680731 5 Grama Panchayat Alur 10 22 A Chalakudy Aloor LALITHA Complete loss of Buildings Nayathodan House,Thiruthiparambu,Near 6 Grama Panchayat Alur 10 89 Chalakudy Aloor Kochamma Thiruthiparambu Church,Annalloor,po Complete loss of Buildings Vellanchira,Thrissur,Kerala,680697 7 Grama Panchayat Alur 11 157 Chalakudy Aloor Leela Koodoly house Karoor P O PIN 680697 Complete loss of Buildings Thooyath Vallakkunu Kallettumkara 8 Grama Panchayat Alur 23 441 Chalakudy Kallettumkara Sunil T.G Complete loss of Buildings Thrissur 680683 KATTEMKULAM KALLETTUMKARA P.O 9 Grama Panchayat Alur 23 621 Chalakudy Kallettumkara MINI SIVAN Complete loss of Buildings THRISSUR PIN:680683 10 Grama Panchayat Annamanada 1 71 Chalakudy Alathur Xavier T. T Themaliparambil house, -

Industrial Profile- Thrissur District 2016-2017
Government of India Ministry of MSME Brief Industrial Profile of Thrissur District 2016-17 Carried out by MSME – Development Institute, Thrissur Ayyanthole P.O., Kanjani Road, Kerala – 680003 Email: [email protected] Website: www.msmsedithrissur.gov.in Phone: 0487-2360536, Fax: 0487-2360216 Brief Industrial Profile of Thrissur District, 2016-17 FOREWORD MSME-DI, Thrissur, as part of its activities has prepared the District Industrial Profile of Thrissur District during 2016-17. The objective to prepare the Industrial Profile of Thrissur is to make the information readily available in respect of general characteristics of the district, administrative set up, industrial scenario, potential for new MSMEs, details of existing clusters and general issues raised by industry associations etc. to the existing as well as prospective entrepreneurs. It is expected that this Industrial Profile will be useful for Governmental & Non-Governmental organizations, Promotional & Developmental Agencies engaged in promotion & development of MSME sector in Thrissur District and also for academicians / research scholars. I take this opportunity to express my gratitude to District Industries Centre, Thrissur, officials of State Planning Board, Economic and Statistics Department, Kerala, Department of Mines and Geology and heads of other departments for extending their co-operation in preparing this Industrial Profile. I also place on records my appreciation to Smt. Kathreenamma Sebastian, Asst Director Gr.I (E.I.) and Smt. Lachithamol U.C., Asst Director (E.I.) of this institute for their hard work in bringing out the report in this form. Thrissur 31.03.2017 MSME- Development Institute, Thrissur 2 Brief Industrial Profile of Thrissur District, 2016-17 Contents Sl. -
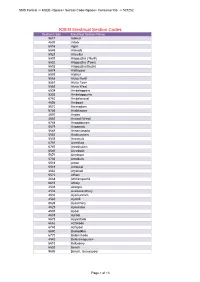
KSEB Electrical Section Codes
SMS Format -> KSEB <Space> Section Code<Space> Consumer No -> 537252 KSEB Electrical Section Codes Section Code Electrical Section Name 5617 Adimali 4609 Adoor 6516 Agali 6648 Alakode 6521 Alanellur 5501 Alappuzha ( North) 5502 Alappuzha (Town) 5503 Alappuzha(South) 6574 Alathiyoor 6509 Alathur 5568 Aluva North 5567 Aluva Town 5569 Aluva West 6534 Ambalappara 5505 Ambalappuzha 6762 Ambalavayal 4656 Amboori 5670 Ammadam 6756 Anakkayam 4597 Anchal 4669 Anchal (West) 6748 Angadipuram 5579 Angamaly. 5648 Annamanada 5553 Arakkunnam 5535 Aranmula 6789 Areekkad 6767 Areekkulam 6549 Areekode 5679 Arimboor 5705 Arookutty 5515 Aroor 5518 Arthinkal 4652 Aryanad 5572 Athani 4664 Athirampuzha 6615 Atholy 4531 Attingal. 4532 Avanavanchery. 4632 Ayarkunnam 4565 Ayathil. 6624 Ayenchery 4629 Aymanam 4591 Ayoor 4608 Ayroor 5678 Ayyanthole 6662 Azhikode 6743 Azhiyoor 6690 Badiadkka 6773 Balamthode 4542 Balaramapuram 6613 Balussery 6603 Beach 5699 Beach, Guruvayoor Page 1 of 13 SMS Format -> KSEB <Space> Section Code<Space> Consumer No -> 537252 4513 Beach, Trivandrum 6635 Beypore 5627 Bharananganam 6697 Bheemanady 5702 Big Bazar 6518 Bigbazar 6655 Burnassery 4558 Cantonment, Kollam 4506 Cantonment. 6602 Central, Kozhikode 4672 Chadayamangalam 6660 Chakkarakkal 6716 Chakkittapara 5651 Chalakudy 6538 Chalissery 6661 Chalode 5508 Champakulam 4636 Changanacherry 6585 Changaramkulam 6745 Chapparappadavu 5527 Charummood 4575 Chathannoor. 6772 Chattanchal 6708 Chattiparamba 5698 Chavakkad 4571 Chavara. 5691 Chelakkara 6744 Chelannur 6577 Chelari 6721 Chemperi 4644 -

Farm Guide 2018
FARM GUIDE 2018 Printed & Published by V. SUMA PRINCIPAL INFORMATION OFFICER FARM INFORMATION BUREAU Kowdiar P.O., Thiruvananthapuram - 695 003 Fax. 0471 - 2318186 e-mail : [email protected]/ [email protected] Compiled and Edited by B. Neena Asst. Director (Technical Asst. IT Division), Dept. of Agriculture Dr. Suja Mary Koshy Editor, Farm News, Dept. of Animal Husbandry Dr. Geetha Ram Information Officer, Dept. of Animal Husbandry Dr. P. Selvakumar Campaign Officer, Dept. of Animal Husbandry Anitha C.S. Agricultural Officer, Dept. of Agriculture Vishnu S.P. Agricultural Officer, Dept. of Agriculture Publication Officer Elizabeth George Asst. Director, Dairy Development Dept. Design & Layout Deepak Mouthatil Articles for Kerala Karshakan: [email protected] 0471- 2314358 Press Release: [email protected] 0471- 2317314 Farm News & General Communication: [email protected] 0471- 2318186 Website: www.fibkerala.gov.in Im¿jnI hnI-k\ I¿jIt£a hIp∏v ktµiw tIcfsaßpw lcnX{]Xo£IfpW¿Øn ]pXph¿jw ho≠pw kamKXamhpIbmWv. Im¿jnItIcfØn\v A`nam\apb¿Øp∂ t\´ßƒ ssIhcn®psIm≠v kwÿm\k¿°mcpw Im¿jnIhnIk\ I¿jIt£a hIp∏pw kZv`cWØns‚ ]mXbn¬ apt∂dpIbmWv. A[nImcta‰v c≠ph¿jw ]q¿Ønbm°p∂ Cu thfbn¬ tZiobXeØn¬Øs∂ {it≤bamb \nch[n I¿jIt£a Im¿jnI˛]cnÿnXnkulrZ ]≤XnIƒ s]mXpP\ ]¶mfnØ tØmsS \n¿hln°m≥Ign™p F∂Xn¬ Gsd NmcnXm¿Yyhpw A`nam\hpap≠v. tIcfØns‚ {][m\ Im¿jnIhnfIfmb s\√pw sXßpw C∂v kwÿm\k¿°m¿ ]≤XnIfneqsS Xncn®phchns‚ ]mXbnemWv. CXns‚ `mKambn k¿°m¿ {]Jym]n® s\¬h¿jmNcWØn\v anI® {]XnIcWhpw ]n¥pWbpamWv e`n®Xv. CXns\ ]n¥pS¿∂v 1193 Nnßw H∂papX¬ 1194 Nnßw H∂phsc tIch¿jambn BNcn®v IrjnhIp∏v hnhn[]≤XnIƒ \S∏nem°pIbmWv.