National Seminar Pharmaceutical Profession and Research in India
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Seat Matrix JELET-2020.Xlsx Page 1 of 33 Seat Matrix, JELET-2020 Counselling
Seat Matrix, JELET-2020 Counselling Institute Name Branch Name OPNO OPPH BANO BAPH BBNO BBPH SCNO SCPH STNO STPH Total Cooch Behar Government Civil Engineering 9 1 2 0 1 0 4 0 1 018 Engineering College, Cooch Behar Cooch Behar Government Computer Science & 5 0 1 0 1 0 1 1 1 010 Engineering College, Cooch Behar Engineering/ Computer Science & Technology Cooch Behar Government Electronics & Communication 8 0 1 1 1 0 3 0 1 015 Engineering College, Cooch Behar Engineering/Electronics & Telecommunication Engineering Cooch Behar Government Electrical Engineering 10 1 2 0 1 0 5 0 1 0 20 Engineering College, Cooch Behar Cooch Behar Government Mechanical Engineering 11 1 2 0 2 0 5 0 1 0 22 Engineering College, Cooch Behar Goverment College of Engineering Computer Science & 11 1 2 0 2 0 5 0 1 0 22 and Leather Technology, Kolkata Engineering/ Computer Science & Technology Goverment College of Engineering Information Technology 5 0 1 0 1 0 2 0 1 010 and Leather Technology, Kolkata Goverment College of Engineering Leather Technology 8 1 2 0 1 0 4 0 1 017 and Leather Technology, Kolkata Govt. College of Engg. & Textile Computer Science & 11 Technology, Berhampore Engineering/ Computer Science 6 1 1 0 1 0 1 0 1 0 & Technology Govt. College of Engg. & Textile Electrical Engineering 6 Technology, Berhampore 3 0 1 0 0 0 1 0 1 0 Govt. College of Engg. & Textile Mechanical Engineering 10 Technology, Berhampore 5 0 1 0 1 0 2 0 1 0 Govt. College of Engg. & Textile Textile Technology 14 Technology, Berhampore 7 1 1 0 1 0 2 1 1 0 Govt. -

Maulana Abul Kalam Azad University of Technology, West Bengal Office of the Information Scientist NH-12, Haringhata, Simhat - 741249
Maulana Abul Kalam Azad University of Technology, West Bengal Office of the Information Scientist NH-12, Haringhata, Simhat - 741249 No. - 001/AISHE/IS/2020/6 Date 19-Feb-2020 Notice To The Principal/Director All Affiliated Colleges of MAKAUT,WB Sub: Uploading of mandatory survey data for 2019-20 on AISHE portal This is for your kind information that uploading of survey data for AISHE 2019-20 has been started. The colleges / institutions affiliated to MAKAUT,WB are requested to upload DCF-II by 21st February, 2020 and in case of any problem write to [email protected] . The following newly affiliated colleges are requested to include their college names, register a nodal officer and upload DCF-II in the AISHE portal. Sr. Name of the college 1 Anand College of Education 2 WEBEL DQE Animation Academy 3 River Research Institute 4 Susrut Eye Foundation & Research Centre 5 Ghani Khan Choudhury Institute of Engineering and Technology 6 Subhas Bose Institute of Hotel Management The following colleges are requested upload DCF-II in the AISHE portal . St at Sr. Login Nodal Officer Name of the college us 1 ABACUS240 Bijayde ABACUS Institute of Engineering and Management 240 done 2 abs216 Bratatikundu ABS Academy of Science, Technology and Management 216 3 ABS Bratatikundu ABS Academy of Science, Technology and Management 328 4 aot169 Dr. Amitavasil Academy of Technology 169 5 aims123 Ramkrishnamondal Advanced Information and Management Studies 198 done 6 aimkolkata Majumdarmalini Army Institute of Management 136 done 7 aiemd4mgt Swarup Choudhury Aryabhatta Institute of Engg. and Management 327 done 8 aecregistrar Dr. -

Private Engineering College Libraries in West Bengal : an Overview
International Journal of Interdisciplinary and Multidisciplinary Studies (IJIMS), 2017, Vol 4, No.3,412-418. 412 Available online at http://www.ijims.com ISSN - (Print): 2519 – 7908 ; ISSN - (Electronic): 2348 – 0343 IF:4.335; Index Copernicus (IC) Value: 60.59; UGC Recognized -UGC Journal No.: 47192. 1st July Private Engineering College Libraries in West Bengal : An overview Biswajit Das 1* and Juran Krishna Sarkhel 2 1.Assistant Librarian, Neotia Institute of Technology, Management and Science Diamond Harbour Road, India 2. Professor, DLIS, Kalyani University, India *Corresponding Author: Biswajit Das Abstract We are focus on the Engineering Education in India, Engineering College Library, Private Engineering College Libraries of West Bengal. We are given a very brief description on each and every private engineering college. Keywords : Engineering Education, Engineering College, Private Engineering College Libraries, West Bengal, India Introduction The unbelievable advancement of science and complexity of technology are making technical education more and more complex and interdisciplinary in nature. Technical education is a tailor-made learning-training system. It is specifically designed for the supply of trained manpower for industrial and economic development through the judicious application of science and technology.[7] Engineering Education in India Engineering education in India started during the British era and mainly focused on civil engineering.[1] The British rulers set up four engineering colleges in the four corners of -
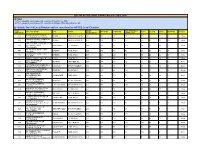
LIST of ACTIVE LOCAL CHAPTERS (Jan
LIST OF ACTIVE LOCAL CHAPTERS (Jan - April 2019) CRITERIA 1) There should be a minimum of 25 exam takers from the LC, AND 2) There should be a minimum of 13 exam takers getting certified (Overall Score>40) By default, Top 100 Local Chapters will be considered as ACTIVE Local Chapters. LOCAL EXAM SUCCESSFULLY COLLEGE NAME CITY STATE PRESENT CERTIFIED ELITE SILVER GOLD TOPPERS STATUS CHAPTER ID REGISTERED COMPLETED ACROPOLIS INSTITUTE OF 422 INDORE MADHYA PRADESH 265 247 231 82 84 48 17 16 Active TECHNOLOGY & RESEARCH LAKSHMI NARAIN COLLEGE OF 476 BHOPAL MADHYA PRADESH 172 169 154 35 45 42 32 19 Active TECHNOLOGY & SCIENCE VNR VIGNANA JYOTHI INSTITUTE 738 OF ENGINEERING & HYDERABAD TELANGANA 299 274 251 107 79 45 20 13 Active TECHNOLOGY SRI SAI RAM ENGINEERING 1003 CHENNAI TAMIL NADU 200 172 162 42 55 47 18 24 Active COLLEGE PANIMALAR ENGINEERING 161 CHENNAI TAMIL NADU 279 247 224 89 69 44 22 10 Active COLLEGE 781 KONGU ENGINEERING COLLEGE ERODE TAMIL NADU 191 175 168 47 48 55 18 16 Active MCKV INSTITUTE OF 539 HOWRAH WEST BENGAL 264 244 218 82 71 58 7 15 Active ENGINEERING INSTITUTE OF ENGINEERING & 1568 LUCKNOW UTTAR PRADESH 128 122 121 16 33 58 14 23 Active TECHNOLOGY, LUCKNOW GEETANJALI INSTITUTE OF 415 UDAIPUR RAJASTHAN 292 278 261 129 72 51 9 14 Active TECHNICAL STUDIES KPR INSTITUTE OF 269 ENGINEERING AND COIMBATORE TAMIL NADU 209 198 179 71 44 30 34 13 Active TECHNOLOGY RAJKIYA ENGINEERING 1926 MAINPURI UTTAR PRADESH 222 210 201 46 83 63 9 11 Active COLLEGE, MAINPURI PRAGATI ENGINEERING 389 EAST GODAVARI ANDHRA PRADESH 312 298 274 -
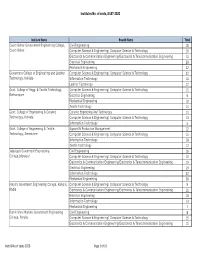
Institutes & No. of Seats, JELET-2020
Institutes No. of seats, JELET-2020 Institute Name Branch Name Total Cooch Behar Government Engineering College, Civil Engineering 18 Cooch Behar Computer Science & Engineering/ Computer Science & Technology 10 Electronics & Communication Engineering/Electronics & Telecommunication Engineering 15 Electrical Engineering 20 Mechanical Engineering 22 Goverment College of Engineering and Leather Computer Science & Engineering/ Computer Science & Technology 22 Technology, Kolkata Information Technology 10 Leather Technology 17 Govt. College of Engg. & Textile Technology, Computer Science & Engineering/ Computer Science & Technology 11 Berhampore Electrical Engineering 6 Mechanical Engineering 10 Textile Technology 14 Govt. College of Engineering & Ceramic Ceramic Enginering And Technology 11 Technology, Kolkata Computer Science & Engineering/ Computer Science & Technology 13 Information Technology 5 Govt. College of Engineering & Textile Apparel & Production Management 12 Technology, Serampore Computer Science & Engineering/ Computer Science & Technology 15 Information Technology 18 Textile Technology 12 Jalpaiguri Goverment Engineering Civil Engineering 16 College,Jalpaiguri Computer Science & Engineering/ Computer Science & Technology 15 Electronics & Communication Engineering/Electronics & Telecommunication Engineering 19 Electrical Engineering 19 Information Technology 22 Mechanical Engineering 20 Kalyani Goverment Engineering College, Kalyani, Computer Science & Engineering/ Computer Science & Technology 9 Nadia Electronics & Communication -
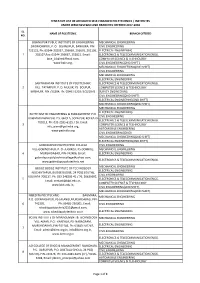
List of Affiliated Self-Financed Polytechnics / Institutes Under Wbsct&Ve&Sd and Branches Offered 2017-2018
TENTATIVE LIST OF AFFILIATED SELF-FINANCED POLYTECHNICS / INSTITUTES UNDER WBSCT&VE&SD AND BRANCHES OFFERED 2017-2018 SL. NAME OF POLYTECNIC BRANCH OFFERED NO. BISHNUPUR PUBLIC INSTITUTE OF ENGINEERING MECHANICAL ENGINEERING SIROMONIPUR, P. O. -BISHNUPUR, BANKURA, PIN- CIVIL ENGINEERING 722122, Ph: 03244-202037, 256669, 256670, 202106, ELECTRICAL ENGINEERING 1 202107,Fax: 03244-256667, 252821, Email: ELECTRONICS & TELECOMMUNICATION ENGG. [email protected], COMPUTER SCIENCE & TECHNOLOGY www.bpie.org, CIVIL ENGINEERING(2ND SHIFT ) MECHANICAL ENGINEERING(2ND SHIFT) CIVIL ENGINEERING MECHANICAL ENGINEERING ELECTRICAL ENGINEERING SANTINIKATAN INSTITUTE OF POLYTECHNIC ELECTRONICS & TELECOMMUNICATION ENGG. 2 VILL- TATARPUR, P. O. MULUK, PS- BOLPUR, COMPUTER SCIENCE & TECHNOLOGY BIRBHUM, PIN 731204, Ph: 03463-221073/221078 SURVEY ENGINEERING CIVIL ENGINEERING(2ND SHIFT) ELECTRICAL ENGINEERING(2ND SHIFT) MECHANICAL ENGINEERING(2ND SHIFT) MECHANICAL ENGINEERING ELECTRICAL ENGINEERING ELITTE INST OF ENGINEERING & MANAGEMENT P.O. CIVIL ENGINEERING KARNAMADHABPUR, P.S. GHOLA, SODEPUR, KOLKATA- ELECTRONICS & TELECOMMUNICATION ENGG. 700113, Ph: 033-2595-6125 / 26, Email: 3 COMPUTER SCIENCE & TECHNOLOGY [email protected], AUTOMOBILE ENGINEERING www.petindia.org CIVIL ENGINEERING(2ND) MECHANICAL ENGINEERING(2ND SHIFT) ELECTRICAL ENGINEERING(2ND SHIFT) GOBINDAPUR POLYTECHNIC COLLEGE CIVIL ENGINEERING VILL-GOBINDAPUR, P. O.-JUGINDA, PS-DOMKAL, MECHANICAL ENGINEERING 4 MURSHIDABAD, PIN-742406, Email: ELECTRICAL ENGINEERING [email protected], -

WBJEE Counselling 2020
WBJEE Counselling 2020 Seat Vacancy after closing of 2nd Cycle institute program Institute Name Branch quota Seat Category Vacancy Id Id 104532 Cooch Behar Government Engineering College, Cooch Behar 10003NN Civil Engineering HS OBC - A (PwD) 1 104532 Cooch Behar Government Engineering College, Cooch Behar 10003NN Civil Engineering HS Open (PwD) 2 104532 Cooch Behar Government Engineering College, Cooch Behar 10013NN Electrical Engineering HS OBC - A 1 104532 Cooch Behar Government Engineering College, Cooch Behar 10013NN Electrical Engineering HS OBC - A (PwD) 1 104532 Cooch Behar Government Engineering College, Cooch Behar 10013NN Electrical Engineering HS OBC - B (PwD) 1 104532 Cooch Behar Government Engineering College, Cooch Behar 10013NN Electrical Engineering HS Open 1 104532 Cooch Behar Government Engineering College, Cooch Behar 10013NN Electrical Engineering HS Open (PwD) 1 104532 Cooch Behar Government Engineering College, Cooch Behar 10013NN Electrical Engineering HS ST 2 104532 Cooch Behar Government Engineering College, Cooch Behar 10022NN Mechanical Engineering HS OBC - A 3 104532 Cooch Behar Government Engineering College, Cooch Behar 10022NN Mechanical Engineering HS Open (PwD) 2 104532 Cooch Behar Government Engineering College, Cooch Behar 10022NN Mechanical Engineering HS SC (PwD) 1 104532 Cooch Behar Government Engineering College, Cooch Behar 10022NN Mechanical Engineering HS ST 2 104532 Cooch Behar Government Engineering College, Cooch Behar 11638NN Computer Science & Engineering/ Computer Science & Technology HS -

Colleges Coming Under Each Nodal Center
Notice from MAKAUT, West Bengal Date : To: The Principal of MAKAUT,WB©s Affiliated Colleges Nominated as Nodal Centres (NC) for Spoken Tutorial Training Program Since 2012 Maulana Abul Kalam Azad University of Technology, West Bengal (MAKAUT,WB), formerly WBUT has taken the lead to promote and enable this ICT effort through Spoken Tutorial (ST) in our departments and affiliated colleges in the State. In continuation, we have nominated 5 Affiliated Colleges region-wise as “Nodal Centres” (NC) for Spoken Tutorial (ST) which have been assigned the clusters (colleges coming under each nodal). Please see the cluster list below. List of NC s - 1) JIS College of Engineering 2) Jalpaiguri Govt. Engineering college 3) Asansol Engineering college 4) Supreme Knowledge Foundation Group of Institutions 5) B.P. Poddar Institute of Management and Technology. Nodal Centre Role 1) Complete the work in their own college in the first month of semester starting/ them becoming a NC in the form of preparing a All Semesters Training Plan covering ALL the Departments and Batches and submitting the Semester Training Planner (STP) on ST website (www.spoken- tutorial.org). 2) Then bring in the Cluster colleges. 1. Appoint a Nodal Officer(NO), make a Faculty-Student Team (4-5) and send an official acknowledgement letter to ST, IITB. 2. Register on Spoken Tutorial website to enroll students- by 4th Feb ( Faculty Coordinator Registration Link ) 3. Download courses by 5th Feb (Download the course link ) 4. Upload students data - Master Batch & STP by 5th Feb (Master Batch Upload link ; Semester Training Planner (STP) link ) 5. -

List of Colleges & Its Courses Affiliated by Maulana Abul Kalam Azad
List of Colleges & Its Courses Affiliated by Maulana Abul Kalam Azad University of Technology, West Bengal (formerly West Bengal University of Technology) for the Academic Year 2018-2019 (As on 14.12.2018) SL. MAKAUT,WB COLLEGE NAME COLLEGE ADDRESS LEVEL OF NAME OF COURSE INTAKE AS INTAKE AS NO. COLLEGE COURSE PER PER CODE MAKAUT, MAKAUT, WB 2017-18 WB 2018-19 1 101 JALPAIGURI GOVERNMENT JALPAIGURI GOVT. ENGG UG CIVIL ENGINEERING 60 60 ENGINEERING COLLEGE COLLEGE, JALPAIGURI - 735102 UG COMPUTER SCIENCE AND ENGINEERING 60 60 WEST BENGAL UG ELECTRICAL ENGINEERING 60 60 Tel: (03561) 255132 UG ELECTRONICS & COMMUNICATION ENGG 60 60 Telefax: (03561) 255131 UG INFORMATION TECHNOLOGY 60 60 E-mail: [email protected] UG MECHANICAL ENGINEERING 60 60 PG MECHANICAL ENGINEERING 15 15 PG ELECTRICAL ENGINEERING 15 15 2 102 KALYANI GOVERNMENT ENGINEERING KALYANI UNIVERSITY CAMPUS UG ELECTRICAL ENGINEERING 60 60 COLLEGE KALYANI-741235, DIST. NADIA UG COMPUTER SCIENCE & ENGINEERING 60 60 WEST BENGAL INDIA UG ELECTRONICS AND COMMUNICATIONS 60 60 Tel: (033) 2582-6680 ENGINEERING Telefax: (033) 2582-1309 UG INFORMATION TECHNOLOGY 60 60 E-mail: [email protected] UG MECHANICAL ENGINEERING 60 60 PG COMPUTER SCIENCE & ENGINEERING 18 18 PG ELECTRONICS AND COMMUNICATIONS 18 18 ENGINEERING PG PRODUCTION ENGINEERING 18 18 PG ELECTRICAL ENGINEERING 18 18 PG INFORMATION TECHNOLOGY 18 18 PG MASTERS IN COMPUTER APPLICATIONS 40 40 3 103 HALDIA INSTITUTE OF TECHNOLOGY ICARE COMPLEX; HIT CAMPUS, UG APPLIED ELECTRONICS AND 60 60 HALDIA, PURBA MEDINIPUR, INSTRUMENTATION -
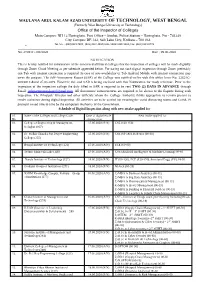
NOTIFICATION-Digital Inspection of Affiliated Colleges -09-06-2020
MAULANA ABUL KALAM AZAD UNIVERSITY OF TECHNOLOGY, WEST BENGAL (Formerly West Bengal University of Technology) Office of the Inspector of Colleges Main Campus: NH 12,Haringhata, Post Office – Simhat, Police Station – Haringhata, Pin - 741249 City Campus: BF-142, Salt Lake City, Kolkata – 700 064 Tel. No. : (033)2321-7588, (033) 2334-1014/1021/1025/1028/1031; Fax : (033) 2321-8776 _________________________________________________________________________________________________________________________________ No. 2709/IC-188/2020 Date : 09.06.2020 NOTIFICATION This is hereby notified for information of the concerned affiliated colleges that the inspection of colleges will be made digitally through Zoom Cloud Meeting as per schedule appended below. For caring out such digital inspection through Zoom preferably one Tab with internet connection is required. In case of non-availability of Tab Android Mobile with internet connection may serve the purpose. The Self-Assessment Report (SAR) of the College was notified earlier vide this office letter No: 2365/IC- 209/2019 dated 25.03.2019. However, the said SAR is being enclosed with this Notification for ready reference. Prior to the inspection of the respective college the duly filled in SAR is required to be sent TWO (2) DAYS IN ADVANCE through Email: [email protected]. All documents/ infrastructures are required to be shown to the Experts during such inspection. The Principal/ Director and other Officials whom the College Authority thinks appropriate to remain present to render assistance during digital inspection. All activities are to be carried out ensuring the social distancing norms and Covid-19 protocol issued time to time by the competent Authority of the Government. Schedule of Digital Inspection along with new intake applied for Sl. -

0-Nodal-Centre-Final-1.Pdf
No:23.6/Regis/NC/2017 Date: 22/06/2017 To The Principal / Director All Affiliated Colleges/Institutes Dear Sir/Madam, The University has decided to organize the following activities through the Nodal Centres amongst the affiliated colleges of University as per the schedule of the academic year 2017-2018 given in the Table below: (a) Remedial classes for the weak students; (b) Training and placement related activities; (c) Awareness for start-up and incubation; (d) Assistance for NBA Accreditation Programmes; (e) Workshops /Seminars/Special classes/Teacher’s Training /Organization of students' competition, etc. One or more of these activities will be carried out in the respective Nodal Centres. The colleges situated in Nodal Centre Liaison incharge Contact person with phone no. Districts from University Zone one-1 Siliguri Dr.Indranil Prof. J.Jhampati(Vice Chairman) Jalpaiguri, Darjeeling, Institute of Mukherjee Dr. Banani Adhikary(Coordinator) Dinajpur(North & South), Technology 9434352534 Malda,Alipurduar ,Coochbehar Zone Two 2: B.C.Ray Engg Dr.Debasish De Prof. A.Sinha( Vice Chairman) Bankura , Birbhum Burdwan College Prof. Avijit Bhowmik, (Coordinator) and .Purulia 94333 54734 [email protected] Zone Three 3:- JIS college of Mr.Bivash Mallick Prof. Malay R Dave( Vice Chairman) Murshidabad, Nadia and North Engg 24 Parganas Zone Four 4:- Dr. Madhumita Kolkata Municipal Corporation Das Sarkar area,Bidhan Nagar Municipal & corporation area and South 24 Dr. Suparna Parganas Biswas Zone -5 Supreme Dr.Santunu Prof ( Dr) Amit Kumar Aditya(Vice Hooghly, Howrah, East Knowledge Phadikar Chairman) 033-26831141,8335056828 Midnapur, & West Midnapur Foundation Group of Institutions, Hooghly The meeting will finalise the modalities for organizing the various activities as well as form a Zonal Organizing committee. -

Page 1 1 KAZI NAZRUL UNIVERSITY ASANSOL
KAZI NAZRUL UNIVERSITY ASANSOL --------------------------------------------------------------------------------------- Dr. ARINDAM BISWAS Assistant Professor and Coordinator, Department of Mining Engineering Coordinator (Addl. Charge), Department of Computer Science. Member, CENTRE FOR ORGANIC SPINTRONICS AND OPTOELECTRONICS DEVICES (COSOD) Course Coordinator, Electronics (UG Studies) Kazi Nazrul University (Public University), Asansol , West Bengal, India Email: [email protected] Phone: +919932311710 Short Biography: Dr. Arindam BISWAS was born in West Bengal, India in 1984. He received M-Tech degree in Radio Physics and Electronics from University of Calcutta, India in 2010 and PhD from NIT Durgapur in 2013. He has worked as a Post-Doctoral Researcher at Pusan National University, South Korea with prestigious BK21PLUS Fellowship, Republic of Korea 2015, DST-JSPS Invitation Research Grant Award in 2020. He got Visiting Professor at Research Institute of Electronics, Shizouka University, Japan. He has been selected for IE(I) Young Engineer Award : 2019-20 in Electronics & Telecommunication Engineering discipline, Institute of Engineers, India. Dr. Biswas has 12 years’ of experience in teaching research and administration. Presently Dr. Biswas is working as an Assistant Professor in School of Mines and Metallurgy at Kazi Nazrul University, Asansol, WB, India. He has 52 technical paper in different journals and 35 conference proceedings and 7 books, 03 Edited Volume and 01 book chapter with international repute. Dr. Biswas received research grant from Science and Engineering Research Board , Govt of India, under Early Career Research Scheme for research in Terahertz based GaN Source. He has also received Research Grant from Centre of Biomedical Engineering, Tokoyo Medical and Dental University in association with RIE, Shizouka University, Japan for study of biomedical THz Imaging based on WBG semiconductor IMPATT Source for two consecutive years 2019-20 and 2020- 2021.