OR52N2 (NM 001005174) Human Tagged ORF Clone Lentiviral Particle Product Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WO 2019/068007 Al Figure 2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2019/068007 Al 04 April 2019 (04.04.2019) W 1P O PCT (51) International Patent Classification: (72) Inventors; and C12N 15/10 (2006.01) C07K 16/28 (2006.01) (71) Applicants: GROSS, Gideon [EVIL]; IE-1-5 Address C12N 5/10 (2006.0 1) C12Q 1/6809 (20 18.0 1) M.P. Korazim, 1292200 Moshav Almagor (IL). GIBSON, C07K 14/705 (2006.01) A61P 35/00 (2006.01) Will [US/US]; c/o ImmPACT-Bio Ltd., 2 Ilian Ramon St., C07K 14/725 (2006.01) P.O. Box 4044, 7403635 Ness Ziona (TL). DAHARY, Dvir [EilL]; c/o ImmPACT-Bio Ltd., 2 Ilian Ramon St., P.O. (21) International Application Number: Box 4044, 7403635 Ness Ziona (IL). BEIMAN, Merav PCT/US2018/053583 [EilL]; c/o ImmPACT-Bio Ltd., 2 Ilian Ramon St., P.O. (22) International Filing Date: Box 4044, 7403635 Ness Ziona (E.). 28 September 2018 (28.09.2018) (74) Agent: MACDOUGALL, Christina, A. et al; Morgan, (25) Filing Language: English Lewis & Bockius LLP, One Market, Spear Tower, SanFran- cisco, CA 94105 (US). (26) Publication Language: English (81) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of national protection available): AE, AG, AL, AM, 62/564,454 28 September 2017 (28.09.2017) US AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, 62/649,429 28 March 2018 (28.03.2018) US CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, (71) Applicant: IMMP ACT-BIO LTD. -

Evaluating Recovery Potential of the Northern White Rhinoceros from Cryopreserved Somatic Cells
Downloaded from genome.cshlp.org on September 26, 2021 - Published by Cold Spring Harbor Laboratory Press Research Evaluating recovery potential of the northern white rhinoceros from cryopreserved somatic cells Tate Tunstall,1 Richard Kock,2 Jiri Vahala,3 Mark Diekhans,4 Ian Fiddes,4 Joel Armstrong,4 Benedict Paten,4 Oliver A. Ryder,1,5 and Cynthia C. Steiner1,5 1San Diego Zoo Institute for Conservation Research, Escondido, California 92027, USA; 2Royal Veterinary College, University of London, London NW1 0TU, United Kingdom; 3Dvur Krlov Zoo, Dvr Krlov nad Labem 544 01, Czech Republic; 4Jack Baskin School of Engineering, University California Santa Cruz, Santa Cruz, California 95064, USA The critically endangered northern white rhinoceros is believed to be extinct in the wild, with the recent death of the last male leaving only two remaining individuals in captivity. Its extinction would appear inevitable, but the development of advanced cell and reproductive technologies such as cloning by nuclear transfer and the artificial production of gametes via stem cells differentiation offer a second chance for its survival. In this work, we analyzed genome-wide levels of genetic diversity, inbreeding, population history, and demography of the white rhinoceros sequenced from cryopreserved somatic cells, with the goal of informing how genetically valuable individuals could be used in future efforts toward the genetic res- cue of the northern white rhinoceros. We present the first sequenced genomes of the northern white rhinoceros, which show relatively high levels of heterozygosity and an average genetic divergence of 0.1% compared with the southern sub- species. The two white rhinoceros subspecies appear to be closely related, with low genetic admixture and a divergent time <80,000 yr ago. -

Genetic and Genomics Laboratory Tools and Approaches
Genetic and Genomics Laboratory Tools and Approaches Meredith Yeager, PhD Cancer Genomics Research Laboratory Division of Cancer Epidemiology and Genetics [email protected] DCEG Radiation Epidemiology and Dosimetry Course 2019 www.dceg.cancer.gov/RadEpiCourse (Recent) history of genetics 2 Sequencing of the Human Genome Science 291, 1304-1351 (2001) 3 The Human Genome – 2019 • ~3.3 billion bases (A, C, G, T) • ~20,000 protein-coding genes, many non-coding RNAs (~2% of the genome) • Annotation ongoing – the initial sequencing in 2001 is still being refined, assembled and annotated, even now – hg38 • Variation (polymorphism) present within humans – Population-specific – Cosmopolitan 4 Types of polymorphisms . Single nucleotide polymorphisms (SNPs) . Common SNPs are defined as > 5% in at least one population . Abundant in genome (~50 million and counting) ATGGAACGA(G/C)AGGATA(T/A)TACGCACTATGAAG(C/A)CGGTGAGAGG . Repeats of DNA (long, short, complex, simple), insertions/deletions . A small fraction of SNPs and other types of variation are very or slightly deleterious and may contribute by themselves or with other genetic or environmental factors to a phenotype or disease 5 Different mutation rates at the nucleotide level Mutation type Mutation rate (per generation) Transition on a CpG 1.6X10-7 Transversion on a CpG 4.4X10-8 Transition: purine to purine Transition out of CpG 1.2X10-8 Transversion: purine to pyrimidine Transversion out of CpG 5.5X10-9 Substitution (average) 2.3X10-8 A and G are purines Insertion/deletion (average) 2.3X10-9 C and T are pyrimidines Mutation rate (average) 2.4X10-8 . Size of haploid genome : 3.3X109 nucleotides . -

Genetic Characterization of Greek Population Isolates Reveals Strong Genetic Drift at Missense and Trait-Associated Variants
ARTICLE Received 22 Apr 2014 | Accepted 22 Sep 2014 | Published 6 Nov 2014 DOI: 10.1038/ncomms6345 OPEN Genetic characterization of Greek population isolates reveals strong genetic drift at missense and trait-associated variants Kalliope Panoutsopoulou1,*, Konstantinos Hatzikotoulas1,*, Dionysia Kiara Xifara2,3, Vincenza Colonna4, Aliki-Eleni Farmaki5, Graham R.S. Ritchie1,6, Lorraine Southam1,2, Arthur Gilly1, Ioanna Tachmazidou1, Segun Fatumo1,7,8, Angela Matchan1, Nigel W. Rayner1,2,9, Ioanna Ntalla5,10, Massimo Mezzavilla1,11, Yuan Chen1, Chrysoula Kiagiadaki12, Eleni Zengini13,14, Vasiliki Mamakou13,15, Antonis Athanasiadis16, Margarita Giannakopoulou17, Vassiliki-Eirini Kariakli5, Rebecca N. Nsubuga18, Alex Karabarinde18, Manjinder Sandhu1,8, Gil McVean2, Chris Tyler-Smith1, Emmanouil Tsafantakis12, Maria Karaleftheri16, Yali Xue1, George Dedoussis5 & Eleftheria Zeggini1 Isolated populations are emerging as a powerful study design in the search for low-frequency and rare variant associations with complex phenotypes. Here we genotype 2,296 samples from two isolated Greek populations, the Pomak villages (HELIC-Pomak) in the North of Greece and the Mylopotamos villages (HELIC-MANOLIS) in Crete. We compare their genomic characteristics to the general Greek population and establish them as genetic isolates. In the MANOLIS cohort, we observe an enrichment of missense variants among the variants that have drifted up in frequency by more than fivefold. In the Pomak cohort, we find novel associations at variants on chr11p15.4 showing large allele frequency increases (from 0.2% in the general Greek population to 4.6% in the isolate) with haematological traits, for example, with mean corpuscular volume (rs7116019, P ¼ 2.3 Â 10 À 26). We replicate this association in a second set of Pomak samples (combined P ¼ 2.0 Â 10 À 36). -

Table S3. RAE Analysis of Well-Differentiated Liposarcoma
Table S3. RAE analysis of well-differentiated liposarcoma Model Chromosome Region start Region end Size q value freqX0* # genes Genes Amp 1 145009467 145122002 112536 0.097 21.8 2 PRKAB2,PDIA3P Amp 1 145224467 146188434 963968 0.029 23.6 10 CHD1L,BCL9,ACP6,GJA5,GJA8,GPR89B,GPR89C,PDZK1P1,RP11-94I2.2,NBPF11 Amp 1 147475854 148412469 936616 0.034 23.6 20 PPIAL4A,FCGR1A,HIST2H2BF,HIST2H3D,HIST2H2AA4,HIST2H2AA3,HIST2H3A,HIST2H3C,HIST2H4B,HIST2H4A,HIST2H2BE, HIST2H2AC,HIST2H2AB,BOLA1,SV2A,SF3B4,MTMR11,OTUD7B,VPS45,PLEKHO1 Amp 1 148582896 153398462 4815567 1.5E-05 49.1 152 PRPF3,RPRD2,TARS2,ECM1,ADAMTSL4,MCL1,ENSA,GOLPH3L,HORMAD1,CTSS,CTSK,ARNT,SETDB1,LASS2,ANXA9, FAM63A,PRUNE,BNIPL,C1orf56,CDC42SE1,MLLT11,GABPB2,SEMA6C,TNFAIP8L2,LYSMD1,SCNM1,TMOD4,VPS72, PIP5K1A,PSMD4,ZNF687,PI4KB,RFX5,SELENBP1,PSMB4,POGZ,CGN,TUFT1,SNX27,TNRC4,MRPL9,OAZ3,TDRKH,LINGO4, RORC,THEM5,THEM4,S100A10,S100A11,TCHHL1,TCHH,RPTN,HRNR,FLG,FLG2,CRNN,LCE5A,CRCT1,LCE3E,LCE3D,LCE3C,LCE3B, LCE3A,LCE2D,LCE2C,LCE2B,LCE2A,LCE4A,KPRP,LCE1F,LCE1E,LCE1D,LCE1C,LCE1B,LCE1A,SMCP,IVL,SPRR4,SPRR1A,SPRR3, SPRR1B,SPRR2D,SPRR2A,SPRR2B,SPRR2E,SPRR2F,SPRR2C,SPRR2G,LELP1,LOR,PGLYRP3,PGLYRP4,S100A9,S100A12,S100A8, S100A7A,S100A7L2,S100A7,S100A6,S100A5,S100A4,S100A3,S100A2,S100A16,S100A14,S100A13,S100A1,C1orf77,SNAPIN,ILF2, NPR1,INTS3,SLC27A3,GATAD2B,DENND4B,CRTC2,SLC39A1,CREB3L4,JTB,RAB13,RPS27,NUP210L,TPM3,C1orf189,C1orf43,UBAP2L,HAX1, AQP10,ATP8B2,IL6R,SHE,TDRD10,UBE2Q1,CHRNB2,ADAR,KCNN3,PMVK,PBXIP1,PYGO2,SHC1,CKS1B,FLAD1,LENEP,ZBTB7B,DCST2, DCST1,ADAM15,EFNA4,EFNA3,EFNA1,RAG1AP1,DPM3 Amp 1 -

Contents 1 Northern White Rhinoceros Pedigree
Contents 1 Northern white rhinoceros pedigree 1 2 Genetic Divergence 2 3 Shared SNP Polymorphism 2 4 Admixture and PCA 4 5 Mitochondrial Tree 5 6 Demographic Inference Using @a@i 7 7 Inbreeding 8 8 Selection 9 9 Identification of the X chromosome 14 1 Northern white rhinoceros pedigree Figure 1: NWR pedigree highlighting individuals sequenced in this study (in blue box) presumably unrelated, with name, studbook number, ID number, and ploidy number 1 Dinka Lucy Angalifu Nola Nadi SB #74 SB #28 SB #348 SB #374 SB #376 KB6571- KB3731- KB9947- KB 8175 KB 5764 1845 618 3803 FZ 2859 FZ 1329 2n = 82 2n = 82 2n = 82 2n = 82 2n = 82 Nasima Saut SB #351 SB #373 KB8174- KB9939- 2872 3799 2n = 82 2n = 82 Nabire Suni SB #789 SB #630 KB8172- KB5765- 2864 2863 2n = 81 2n = 82 2 Genetic Divergence Pair-wise genetic divergence was estimated between all pairs of individuals using sites callable among all individuals, and defined as (2*homs+hets)/(2*callable fraction of genome), as defined in (Prado-Martinez et al., 2013). Divergence values for all individuals are shown in Supplementary Material Table 1. Calcu- lations were performed on the full set of 9.4 million SNPs. 3 Shared SNP Polymorphism In order to calculate shared polymorphism between the NWR and SWR, we took the average polymorphism of all possible combinations of the nine NWR 2 Table 1: Pair-wise genetic divergence for all rhinoceroses included in this study SB 28 SB 377 SB 376 SB 372 SB 156 SB 74 SB 24 SB 147 SB 351 SB 374 SB 373 SB 348 SB 34 SB 28 0 0 0 0 0 0 0 0 0 0 0 0 0 SB 377 0.0020231648 -

The Hypothalamus As a Hub for SARS-Cov-2 Brain Infection and Pathogenesis
bioRxiv preprint doi: https://doi.org/10.1101/2020.06.08.139329; this version posted June 19, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. The hypothalamus as a hub for SARS-CoV-2 brain infection and pathogenesis Sreekala Nampoothiri1,2#, Florent Sauve1,2#, Gaëtan Ternier1,2ƒ, Daniela Fernandois1,2 ƒ, Caio Coelho1,2, Monica ImBernon1,2, Eleonora Deligia1,2, Romain PerBet1, Vincent Florent1,2,3, Marc Baroncini1,2, Florence Pasquier1,4, François Trottein5, Claude-Alain Maurage1,2, Virginie Mattot1,2‡, Paolo GiacoBini1,2‡, S. Rasika1,2‡*, Vincent Prevot1,2‡* 1 Univ. Lille, Inserm, CHU Lille, Lille Neuroscience & Cognition, DistAlz, UMR-S 1172, Lille, France 2 LaBoratorY of Development and PlasticitY of the Neuroendocrine Brain, FHU 1000 daYs for health, EGID, School of Medicine, Lille, France 3 Nutrition, Arras General Hospital, Arras, France 4 Centre mémoire ressources et recherche, CHU Lille, LiCEND, Lille, France 5 Univ. Lille, CNRS, INSERM, CHU Lille, Institut Pasteur de Lille, U1019 - UMR 8204 - CIIL - Center for Infection and ImmunitY of Lille (CIIL), Lille, France. # and ƒ These authors contriButed equallY to this work. ‡ These authors directed this work *Correspondence to: [email protected] and [email protected] Short title: Covid-19: the hypothalamic hypothesis 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.06.08.139329; this version posted June 19, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Influence of Gene Modification in Biological Behaviors and Responses of Mouse Lung Telocytes to Inflammation
Song et al. J Transl Med (2019) 17:158 https://doi.org/10.1186/s12967-019-1870-y Journal of Translational Medicine RESEARCH Open Access Infuence of gene modifcation in biological behaviors and responses of mouse lung telocytes to infammation Dongli Song1†, Menglin Xu1†, Ruixue Qi1†, Ruihua Ma1, Yile Zhou1, Duojiao Wu1,2*, Hao Fang3,4* and Xiangdong Wang1,2* Abstract Background: Telocytes play key roles in maintenance of organ/tissue function and prevention of organ injury. However, there are great challenges to investigate telocytes functions using primary telocytes, due to the difculties of isolation, identifcation, and stability. The present study aims at constructing continuous cell strain of mouse lung telocyte cell line with stable characters by gene modifcation and investigating biological behaviors and responses of gene-modifed telocytes to infammation. Methods: Mouse primary lung telocytes were isolated and identifed using immune-labeling markers and immuno- electron microscopy. Primary telocytes were transformed with Simian vacuolating virus 40 small and large T antigen (SV40). Biological characters, behaviors morphology, and proliferation of those gene-modifed telocytes were defned and monitored dynamically for 50 generations, as compared with primary lung telocytes. Cell cycle of mouse primary lung telocytes or gene-modifed telocytes was detected by fow cytometry. Results: Gene modifed telocytes of generations 5, 10, 30 and 50 were observed with telopodes and also showed CD34 and ckit positive. Multiple cellular morphology were also observed on telocyte cell-line under monitor of celliq and enhanced cell proliferation were showed. SV40 transduction was also reduced apoptosis and increased the ratio of S and G2 phases in telocyte cell-line. -

Supplementary Materialsupplementary Material
DOI:10.1071/AN20275_AC CSIRO 2021 Animal Production Science 2021, 61, 731–744 Genome-wide association study between copy number variation regions and carcass and meat quality traits in Nellore cattle Mariana Piatto Berton1,5, Marcos Vinícius de Antunes Lemos1, Tatiane Cristina Seleguim Chud2, Nedenia Bonvino Stafuzza1, Sabrina Kluska1, Sabrina Thaise Amorim1, Lucas Silva Ferlin Lopes1, Angélica Simone Cravo Pereira3, Derek Bickhart4, George Liu4, Lúcia Galvão de Albuquerque1 and Fernando Baldi1 1Departamento de Zootecnia, Faculdade de Ciências Agrárias e Veterinárias, Universidade Estadual Paulista, Via de acesso Prof. Paulo Donato Castellane, s/no, CEP 14884-900 Jaboticabal, SP, Brazil 2Centre for Genetic Improvement of Livestock, University of Guelph, Guelph, ON, Canada N1G 2W1 3 Faculdade de Zootecnia e Engenharia de Alimentos, Universidade de São Paulo, Rua Duque de Caxias Norte, 225, CEP 13635-900 Pirassununga, SP, Brazil 4 USDA-ARS, ANRI, Bovine Functional Genomics Laboratory, Beltsville, Maryland 20705, USA; 2Department of Animal and Avian Sciences, University of Maryland, College Park, Maryland 20742, USA 5Corresponding author. E-mail: [email protected] Table S1. Description of significant (P value<0.05) CNVRs associated with beef tenderness (BT) and candidate genes within the CNVRs. CNVR ID BTA Type* Start (bp) End (bp) Size (bp) Low** High** P value FDR CNVR_gain_3 1 Gain 1374155 3404571 2030417 27 45 0.026 0.36 CNVR_gain_644 2 Gain 81291862 83154452 1862591 32 15 0.015 0.40 CNVR_loss_936 5 Loss 22514133 22563988 49856 233 -

Output Results of CLIME (Clustering by Inferred Models of Evolution)
Output results of CLIME (CLustering by Inferred Models of Evolution) Dataset: Num of genes in input gene set: 9 Total number of genes: 20834 Prediction LLR threshold: 0 The CLIME PDF output two sections: 1) Overview of Evolutionarily Conserved Modules (ECMs) Top panel shows the predefined species tree. Bottom panel shows the partition of input genes into Evolutionary Conserved Modules (ECMs), ordered by ECM strength (shown at right), and separated by horizontal lines. Each row show one gene, where the phylogenetic profile indicates presence (blue) or absence (gray) of homologs in each species (column). Gene symbols are shown at left. Gray color indicates that the gene is a paralog to a higher scoring gene within the same ECM (based on BLASTP E < 1e-3). 2) Details of each ECM and its expansion ECM+ Top panel shows the inferred evolutionary history on the predefined species tree. Branch color shows the gain event (blue) and loss events (red color, with brighter color indicating higher confidence in loss). Branches before the gain or after a loss are shown in gray. Bottom panel shows the input genes that are within the ECM (blue/white rows) as well as all genes in the expanded ECM+ (green/gray rows). The ECM+ includes genes likely to have arisen under the inferred model of evolution relative to a background model, and scored using a log likelihood ratio (LLR). PG indicates "paralog group" and are labeled alphabetically (i.e., A, B). The first gene within each paralog group is shown in black color. All other genes sharing sequence similarity (BLAST E < 1e-3) are assigned to the same PG label and displayed in gray. -
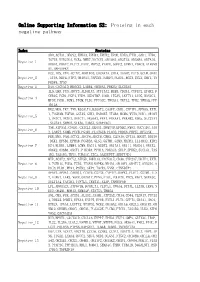
Online Supporting Information S2: Proteins in Each Negative Pathway
Online Supporting Information S2: Proteins in each negative pathway Index Proteins ADO,ACTA1,DEGS2,EPHA3,EPHB4,EPHX2,EPOR,EREG,FTH1,GAD1,HTR6, IGF1R,KIR2DL4,NCR3,NME7,NOTCH1,OR10S1,OR2T33,OR56B4,OR7A10, Negative_1 OR8G1,PDGFC,PLCZ1,PROC,PRPS2,PTAFR,SGPP2,STMN1,VDAC3,ATP6V0 A1,MAPKAPK2 DCC,IDS,VTN,ACTN2,AKR1B10,CACNA1A,CHIA,DAAM2,FUT5,GCLM,GNAZ Negative_2 ,ITPA,NEU4,NTF3,OR10A3,PAPSS1,PARD3,PLOD1,RGS3,SCLY,SHC1,TN FRSF4,TP53 Negative_3 DAO,CACNA1D,HMGCS2,LAMB4,OR56A3,PRKCQ,SLC25A5 IL5,LHB,PGD,ADCY3,ALDH1A3,ATP13A2,BUB3,CD244,CYFIP2,EPHX2,F CER1G,FGD1,FGF4,FZD9,HSD17B7,IL6R,ITGAV,LEFTY1,LIPG,MAN1C1, Negative_4 MPDZ,PGM1,PGM3,PIGM,PLD1,PPP3CC,TBXAS1,TKTL2,TPH2,YWHAQ,PPP 1R12A HK2,MOS,TKT,TNN,B3GALT4,B3GAT3,CASP7,CDH1,CYFIP1,EFNA5,EXTL 1,FCGR3B,FGF20,GSTA5,GUK1,HSD3B7,ITGB4,MCM6,MYH3,NOD1,OR10H Negative_5 1,OR1C1,OR1E1,OR4C11,OR56A3,PPA1,PRKAA1,PRKAB2,RDH5,SLC27A1 ,SLC2A4,SMPD2,STK36,THBS1,SERPINC1 TNR,ATP5A1,CNGB1,CX3CL1,DEGS1,DNMT3B,EFNB2,FMO2,GUCY1B3,JAG Negative_6 2,LARS2,NUMB,PCCB,PGAM1,PLA2G1B,PLOD2,PRDX6,PRPS1,RFXANK FER,MVD,PAH,ACTC1,ADCY4,ADCY8,CBR3,CLDN16,CPT1A,DDOST,DDX56 ,DKK1,EFNB1,EPHA8,FCGR3A,GLS2,GSTM1,GZMB,HADHA,IL13RA2,KIR2 Negative_7 DS4,KLRK1,LAMB4,LGMN,MAGI1,NUDT2,OR13A1,OR1I1,OR4D11,OR4X2, OR6K2,OR8B4,OXCT1,PIK3R4,PPM1A,PRKAG3,SELP,SPHK2,SUCLG1,TAS 1R2,TAS1R3,THY1,TUBA1C,ZIC2,AASDHPPT,SERPIND1 MTR,ACAT2,ADCY2,ATP5D,BMPR1A,CACNA1E,CD38,CYP2A7,DDIT4,EXTL Negative_8 1,FCER1G,FGD3,FZD5,ITGAM,MAPK8,NR4A1,OR10V1,OR4F17,OR52D1,O R8J3,PLD1,PPA1,PSEN2,SKP1,TACR3,VNN1,CTNNBIP1 APAF1,APOA1,CARD11,CCDC6,CSF3R,CYP4F2,DAPK1,FLOT1,GSTM1,IL2 -

Characterization of the Genomic Features and Expressed Fusion Genes In
1 SUPPLEMENTARY INFORMATION (ONLINE SUPPORTING INFORMATION) Characterization of the genomic features and expressed fusion genes in micropapillary carcinomas of the breast Natrajan et al. Supplementary Methods Supplementary Figures S1-S6 Supplementary Tables S1-S7 2 SUPPLEMENTARY METHODS Tumor samples Two cohorts of micropapillary carcinomas (MPCs) were analyzed; the first cohort comprised 16 consecutive formalin fixed paraffin embedded (FFPE) MPCs, 11 pure and 5 mixed, which were retrieved from the authors' institutions (Table 1), and a second, validation cohort comprised 14 additional consecutive FFPE MPCs, retrieved from Molinette Hospital, Turin, Italy. Frozen samples were available from five out of the 16 cases from the first cohort of MPCs. As a comparator for the results of the Sequenom mutation profiling, a cohort of 16 consecutive IC-NSTs matched to the first cohort of 16 MPCs according to ER and HER2 status and histological grade were retrieved from a series of breast cancers previously analyzed by aCGH[1]. In addition, 14 IC-NSTs matched according to grade, and ER and HER2 status to tumors from the second cohort of 14 MPCs, and 48 grade 3 IC-NSTs were retrieved from Hospital La Paz, Madrid, Spain[1] (Supplementary Table S1). Power calculation For power calculations, we have assumed that if MPCs were driven by a recurrent fusion gene in a way akin to secretory carcinomas (which harbor the ETV6-NTRK3 fusion gene in >95% of cases[2-4]) or adenoid cystic carcinomas of the breast (which harbor the MYB-NFIB fusion gene in >90% of cases[5]), a ‘pathognomonic’ driver event would be present in at least ≥70% of cases (an estimate that is conservative).