Knockdown Resistance of Anopheles Sinensis in Henan Province, China
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Exploratory Study of Factors Influencing Examing-Into Xuchang University Ming Guan
Available online at www.sciencedirect.com ScienceDirect Procedia - Social and Behavioral Sciences 116 ( 2014 ) 2664 – 2669 5th World Conference on Educational Sciences - WCES 2013 An exploratory study of factors influencing examing-into Xuchang University Ming Guan School of Economics,Henan University, Kaifeng and 475004,China Xuchang University,, Xuchang and 461000,China Abstract This case study seeks to explain why students choose to pursue advanced education at Xuchang University, and to assess the strengths and dynamics of the factors influencing the enrollment decision. This study used a combination of quantitative and qualitative research methods to explain the factors and process. Quantitative data from a survey questionnaire were used to identify the factors and to measure their significance in influencing or determining the choice of Xuchang University. Qualitative data from in-person interviews were used to gain insights into how freshmen decide to pursue higher education at Xuchang University. The research findings reveal the significant influence of academic, economic, environmental, and job offer /settledown pulling factors as well as a set of negative pushing factors. This research suggests that to attract top freshmen, Xuchang University should focus on investing in research and ensuring the quality of higher education, while crafting a strategy to enhance awareness of and the overall image of their higher education institutions and programs. © 2013 The Authors. Published by Elsevier Ltd. Selection and/or peer-review under responsibility of Academic World Education and Research Center. Keywords: Higher education, enrollment, decision-making, Xuchang University 1. Introduction Xuchang University (XU), located in Xuchang City which is a medium-sized city far from the capital of Henan Province. -

54401-001: Asia Cube Wastewater Treatment Upgrade Project
Environment and Social Compliance Audit Report Project Number: 54401-001 Asset-Level Report - Yongcheng No. 2 April 2021 People’s Republic of China: Asia Cube Wastewater Treatment Upgrade Project Prepared by Stantec Environmental Engineering (Shanghai) Co., Ltd. (“Stantec”) for the China Cube Water Company (the “Client”) and the Asian Development Bank. This environment and social compliance audit report is a document of the borrower. The views expressed herein do not necessarily represent those of ADB's Board of Directors, Management, or staff, and may be preliminary in nature. In preparing any country program or strategy, financing any project, or by making any designation of or reference to a particular territory or geographic area in this document, the Asian Development Bank does not intend to make any judgments as to the legal or other status of any territory or area. ASSET-LEVEL E&S AUDIT REPORT – YONGCHENG NO.2 This document entitled Asset-level E&S audit report – Yongcheng No.2 was prepared by Stantec Environmental Engineering (Shanghai) Co., Ltd. (“Stantec”) for the account of China Cube Water Limited (the “Client”). Any reliance on this document by any third party is strictly prohibited. The material in it reflects Stantec’s professional judgment in light of the scope, schedule and other limitations stated in the document and in the contract between Stantec and the Client. The opinions in the document are based on conditions and information existing at the time the document was published and do not take into account any subsequent changes. In preparing the document, Stantec did not verify information supplied to it by others. -
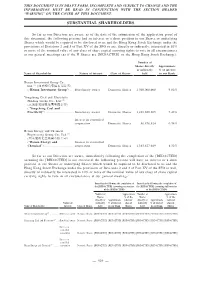
Substantial Shareholders
THIS DOCUMENT IS IN DRAFT FORM, INCOMPLETE AND SUBJECT TO CHANGE AND THE INFORMATION MUST BE READ IN CONJUNCTION WITH THE SECTION HEADED “WARNING” ON THE COVER OF THIS DOCUMENT. SUBSTANTIAL SHAREHOLDERS So far as our Directors are aware, as of the date of the submission of the application proof of this document, the following persons had an interest or a short position in our Shares or underlying Shares which would be required to be disclosed to us and the Hong Kong Stock Exchange under the provisions of Divisions 2 and 3 of Part XV of the SFO or are, directly or indirectly, interested in 10% or more of the nominal value of any class of share capital carrying rights to vote in all circumstances at our general meetings (as if the H Shares are [REDACTED] on the Hong Kong Stock Exchange): Number of Shares directly Approximate or indirectly % of interest Name of Shareholder Nature of interest Class of Shares held in our Bank Henan Investment Group Co., Ltd.(1) (河南投資集團有限公司) (“Henan Investment Group”) .... Beneficiary owner Domestic Shares 1,500,000,000 9.02% Yongcheng Coal and Electricity Holding Group Co., Ltd.(2) (永城煤電控股集團有限公司) (“Yongcheng Coal and Electricity”) .............................. Beneficiary owner Domestic Shares 1,232,960,305 7.42% Interest in controlled corporation Domestic Shares 56,876,624 0.34% Henan Energy and Chemical Engineering Group Co., Ltd.(3) (河南能源化工集團有限公司) (“Henan Energy and Interest in controlled Chemical”)................................. corporation Domestic Shares 1,383,827,049 8.32% So far as our Directors -

Directors, Supervisors and Senior Management
THIS DOCUMENT IS IN DRAFT FORM, INCOMPLETE AND SUBJECT TO CHANGE AND THE INFORMATION MUST BE READ IN CONJUNCTION WITH THE SECTION HEADED “WARNING” ON THE COVER OF THIS DOCUMENT. DIRECTORS, SUPERVISORS AND SENIOR MANAGEMENT BOARD OF DIRECTORS App1A-41(1) The Board consists of eleven Directors, including five executive Directors, two non-executive 3rd Sch 6 Directors and four independent non-executive Directors. The Directors are elected for a term of three years and are subject to re-election, provided that the cumulative term of an independent non-executive Director shall not exceed six years pursuant to the relevant PRC laws and regulations. The following table sets forth certain information regarding the Directors. Time of Time of joining the joining the Thirteen Date of Position held Leading City Time of appointment as of the Latest Group Commercial joining the as a Practicable Name Age Office Banks Bank Director Date Responsibility Mr. DOU 54 December N/A December December Executive Responsible for the Rongxing 2013 2014 23, 2014 Director, overall management, (竇榮興) chairperson of strategic planning and the Board business development of the Bank Ms. HU 59 N/A January 2010 December December Executive In charge of the audit Xiangyun (Joined 2014 23, 2014 Director, vice department, regional (胡相雲) Xinyang chairperson of audit department I and Bank) the Board regional audit department II of the Bank Mr. WANG Jiong 49 N/A N/A December December Executive Responsible for the (王炯) 2014 23, 2014 Director, daily operation and president management and in charge of the strategic development department and the planning and financing department of the Bank Mr. -

How Do Chinese Grassroots Ngos Fight Local Pollution? an Organizational Perspective
How Do Chinese Grassroots NGOs Fight Local Pollution? An Organizational Perspective Yumin Wang Supervisor: Kathinka Fürst, Ph. D Masters project submitted in partial fulfillment of the requirements for the International Master of Environmental Policy at Duke Kunshan University, degree awarded by the Nicholas School of the Environment and Sanford School of Public Policy of Duke University April 25th, 2020 1 How Do Chinese Grassroots NGOs Fight Local Pollution? An Organizational Perspective Abstract In recent years, the Chinese government started to encourage more stakeholders to participate in the environmental protection field. However, little information told us what role grassroots non- governmental organizations (GNGOs) can play in the environmental protection field. In this study, the development history of two GNGOs were traced in parallel. The two GNGOs were both established by environmental journalists who wished to mitigate local pollution. However, the development history of the two GNGOs were totally different. I found that two external factors could explain this difference: government’s different willingness on pollution mitigation and the severity of the pollution. In order to mitigate the local pollution, both of the two GNGOs reached out to different stakeholders, especially government agencies, for cooperation. For the government, this cooperation is beneficial to fulfill their environmental protection responsibility. For the two GNGOs, the cooperation is a stable source of legitimacy, which is critical for GNGO’s survival when they worked on pollution mitigation, a relatively controversial field in China. The cooperation, however, strongly depends on founder’s personal relationship with different stakeholders. This dependency brought both of the two EGNGOs into the trap of elite governance. -

Interim Report 2005 Characteristics of the Growth Enterprise Market (“Gem”) of the Stock Exchange of Hong Kong Limited (The “Stock Exchange”)
(incorporated in the Cayman Islands with limited liability) (Stock Code: 8070) INTERIM REPORT 2005 CHARACTERISTICS OF THE GROWTH ENTERPRISE MARKET (“GEM”) OF THE STOCK EXCHANGE OF HONG KONG LIMITED (THE “STOCK EXCHANGE”) GEM has been established as a market designed to accommodate companies to which a high investment risk may be attached. In particular, companies may list on GEM with neither a track record of profitability nor any obligation to forecast future profitability. Furthermore, there may be risks arising out of the emerging nature of companies listed on GEM and the business sectors or countries in which the companies operate. Prospective investors should be aware of the potential risks of investing in such companies and should make the decision to invest only after due and careful consideration. The greater risk profile and other characteristics of GEM mean that it is a market more suited to professional and other sophisticated investors. Given the emerging nature of companies listed on GEM, there is a risk that securities traded on GEM may be more susceptible to high market volatility than securities traded on the Main Board and no assurance is given that there will be a liquid market in the securities traded on GEM. The principal means of information dissemination on GEM is publication on the internet website at www.hkgem.com operated by the Stock Exchange. Listed companies are not generally required to issue paid announcements in gazetted newspapers. Accordingly, prospective investors should note that they need to have access to the GEM website in order to obtain up-to-date information on GEM-listed issuers. -

Center for Studies in Demography and Ecology
Center for Studies in Demography and Ecology An Evaluation of the One Percent Clustered Sample of the 1990 Census of China by William M. Mason University of California – Los Angeles William Lavely University of Washington UNIVERSITY OF WASHINGTON CSDE Working Paper No. 01-12 An Evaluation of the One Percent Clustered Sample of the 1990 Census of China November 29, 2004 William M. Mason Sociology Department and California Center for Population Research University of California—Los Angeles [email protected] William Lavely Sociology Department and Center for Studies in Demography and Ecology University of Washington [email protected] ABSTRACT We describe and evaluate a one percent clustered sample of the 1990 Census of China, using direct inspection as well as comparisons with published data drawn from the complete enumeration. In the absence of official documentation, we elucidate the basis of the clustering; detect duplicate cases; report corrected totals; and make comparisons between the sample data and tabulations based on the complete enumeration at the province and county levels. Although the sample contains several anomalies, we conclude that it is broadly serviceable. 1. Introduction Two micro-samples of the 1990 Chinese Census have circulated in China and abroad. The first, in order of creation, is a one percent sample of rural administrative villages and urban neighborhoods. (Note 1) The second is a one percent sample of households. We refer to the former, the subject of this article, as the “one percent clustered sample,” and to the latter as the “one percent household sample.” These data sets are not public use micro samples (PUMS) in the sense understood by users of, for example, U.S. -

Enzyme Activity and Morphological Change in the Spleens of Crucian Carp in the Yongcheng Coal Mine Subsidence Area, China
Enzyme activity and morphological change in the spleens of crucian carp in the Yongcheng coal mine subsidence area, China Y.F. Yan1, J.Y. Yang2 and J.Y. Lin2 1School of Life Sciences, Shangqiu Normal University, Shangqiu, Henan, China 2School of Physical Education, Henan Normal University, Xinxiang, Henan, China Corresponding author: J.Y. Yang E-mail: [email protected] Genet. Mol. Res. 15 (2): gmr.15027782 Received December 14, 2015 Accepted January 15, 2016 Published April 26, 2016 DOI http://dx.doi.org/10.4238/gmr.15027782 ABSTRACT. The goal of the current study was to investigate the effects of pollution on aquatic organisms in the Yongcheng coal mine subsidence area. Crucian carp (Carassius auratus) were collected from Yongcheng natural fishpond (experimental group) and Tianmu Lake (control group), and the spleens were isolated for analysis. Subsequently, histological changes, DNA damage, and antioxidant enzyme activity were assessed. The result showed that there were more vacuoles, widened blood sinus cavities, increased partial dot necrosis, and a larger number of brown-yellow nodules in splenic sections stained with hematoxylin and eosin in the experimental group than in the control group. Additionally, it was not easy to distinguish red pulp from white pulp in the experimental group. The antioxidant enzyme activity in the experimental group was significantly lower than that in the control group (P < 0.01). Comet assay results showed varying degrees of tailing and DNA chain breaks in the experimental group, and further analysis demonstrated that the tail length and tail moment Genetics and Molecular Research 15 (2): gmr.15027782 ©FUNPEC-RP www.funpecrp.com.br Y.F. -

Announcement of Annual Results for the Year Ended December 31, 2019
Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this announcement, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this announcement. (Stock Code of H Shares: 1216) (Stock Code of Preference Shares: 4617) ANNOUNCEMENT OF ANNUAL RESULTS FOR THE YEAR ENDED DECEMBER 31, 2019 The board of directors (the “Board”) of Zhongyuan Bank Co., Ltd. (the “Bank”) is pleased to announce the audited consolidated annual results (the “Annual Results”) of the Bank and its subsidiaries for the year ended December 31, 2019 (the “Reporting Period”) which were prepared in accordance with the International Financial Reporting Standards (“IFRSs”). The Board and the audit committee of the Board have reviewed and confirmed the Annual Results. This results announcement is published on the websites of The Stock Exchange of Hong Kong Limited (www.hkexnews.hk) and the Bank (www.zybank.com.cn). The annual report for the year ended December 31, 2019 will be despatched to the shareholders of the Bank and will be available on the above websites in due course. On behalf of the Board Zhongyuan Bank Co., Ltd.* DOU Rongxing Chairman Zhengzhou, the People’s Republic of China March 27, 2020 As at the date of this announcement, the Board comprises Mr. DOU Rongxing, Mr. WANG Jiong, Mr. LI Yulin and Mr. WEI Jie as executive directors; Mr. LI Qiaocheng, Mr. LI Xipeng and Mr. -

Addition of Clopidogrel to Aspirin in 45 852 Patients with Acute Myocardial Infarction: Randomised Placebo-Controlled Trial
Articles Addition of clopidogrel to aspirin in 45 852 patients with acute myocardial infarction: randomised placebo-controlled trial COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group* Summary Background Despite improvements in the emergency treatment of myocardial infarction (MI), early mortality and Lancet 2005; 366: 1607–21 morbidity remain high. The antiplatelet agent clopidogrel adds to the benefit of aspirin in acute coronary See Comment page 1587 syndromes without ST-segment elevation, but its effects in patients with ST-elevation MI were unclear. *Collaborators and participating hospitals listed at end of paper Methods 45 852 patients admitted to 1250 hospitals within 24 h of suspected acute MI onset were randomly Correspondence to: allocated clopidogrel 75 mg daily (n=22 961) or matching placebo (n=22 891) in addition to aspirin 162 mg daily. Dr Zhengming Chen, Clinical Trial 93% had ST-segment elevation or bundle branch block, and 7% had ST-segment depression. Treatment was to Service Unit and Epidemiological Studies Unit (CTSU), Richard Doll continue until discharge or up to 4 weeks in hospital (mean 15 days in survivors) and 93% of patients completed Building, Old Road Campus, it. The two prespecified co-primary outcomes were: (1) the composite of death, reinfarction, or stroke; and Oxford OX3 7LF, UK (2) death from any cause during the scheduled treatment period. Comparisons were by intention to treat, and [email protected] used the log-rank method. This trial is registered with ClinicalTrials.gov, number NCT00222573. or Dr Lixin Jiang, Fuwai Hospital, Findings Allocation to clopidogrel produced a highly significant 9% (95% CI 3–14) proportional reduction in death, Beijing 100037, P R China [email protected] reinfarction, or stroke (2121 [9·2%] clopidogrel vs 2310 [10·1%] placebo; p=0·002), corresponding to nine (SE 3) fewer events per 1000 patients treated for about 2 weeks. -

Results Announcement for the Year Ended December 31, 2020
(GDR under the symbol "HTSC") RESULTS ANNOUNCEMENT FOR THE YEAR ENDED DECEMBER 31, 2020 The Board of Huatai Securities Co., Ltd. (the "Company") hereby announces the audited results of the Company and its subsidiaries for the year ended December 31, 2020. This announcement contains the full text of the annual results announcement of the Company for 2020. PUBLICATION OF THE ANNUAL RESULTS ANNOUNCEMENT AND THE ANNUAL REPORT This results announcement of the Company will be available on the website of London Stock Exchange (www.londonstockexchange.com), the website of National Storage Mechanism (data.fca.org.uk/#/nsm/nationalstoragemechanism), and the website of the Company (www.htsc.com.cn), respectively. The annual report of the Company for 2020 will be available on the website of London Stock Exchange (www.londonstockexchange.com), the website of the National Storage Mechanism (data.fca.org.uk/#/nsm/nationalstoragemechanism) and the website of the Company in due course on or before April 30, 2021. DEFINITIONS Unless the context otherwise requires, capitalized terms used in this announcement shall have the same meanings as those defined in the section headed “Definitions” in the annual report of the Company for 2020 as set out in this announcement. By order of the Board Zhang Hui Joint Company Secretary Jiangsu, the PRC, March 23, 2021 CONTENTS Important Notice ........................................................... 3 Definitions ............................................................... 6 CEO’s Letter .............................................................. 11 Company Profile ........................................................... 15 Summary of the Company’s Business ........................................... 27 Management Discussion and Analysis and Report of the Board ....................... 40 Major Events.............................................................. 112 Changes in Ordinary Shares and Shareholders .................................... 149 Directors, Supervisors, Senior Management and Staff.............................. -

Central China Securities Co., Ltd
Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this announcement, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this announcement. Central China Securities Co., Ltd. (a joint stock company incorporated in 2002 in Henan Province, the People’s Republic of China with limited liability under the Chinese corporate name “中原證券股份有限公司” and carrying on business in Hong Kong as “中州證券”) (Stock Code: 01375) ANNUAL RESULTS ANNOUNCEMENT FOR THE YEAR ENDED 31 DECEMBER 2017 The board (the “Board”) of directors (the “Directors”) of Central China Securities Co., Ltd. (the “Company”) hereby announces the audited annual results of the Company and its subsidiaries for the year ended 31 December 2017. This annual results announcement, containing the full text of the 2017 annual report of the Company, complies with the relevant requirements of the Rules Governing the Listing of Securities on The Stock Exchange of Hong Kong Limited in relation to information to accompany preliminary announcements of annual results and have been reviewed by the audit committee of the Company. The printed version of the Company’s 2017 annual report will be dispatched to the shareholders of the Company and available for viewing on the website of Hong Kong Exchanges and Clearing Limited at www.hkexnews.hk, the website of the Shanghai Stock Exchange at www.sse.com.cn and the website of the Company at www.ccnew.com around mid-April 2018.