Fishes and Decapod Crustaceans of the Great Lakes Basin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Preliminary Assessment of the Native Fish Stocks of Jasper National Park
A Preliminary Assessment of the Native Fish Stocks of Jasper National Park David W. Mayhood Part 3 of a Fish Management Plan for Jasper National Park Freshwater Research Limited A Preliminary Assessment of the Native Fish Stocks of Jasper National Park David W. Mayhood FWR Freshwater Research Limited Calgary, Alberta Prepared for Canadian Parks Service Jasper National Park Jasper, Alberta Part 3 of a Fish Management Plan for Jasper National Park July 1992 Cover & Title Page. Alexander Bajkov’s drawings of bull trout from Jacques Lake, Jasper National Park (Bajkov 1927:334-335). Top: Bajkov’s Figure 2, captioned “Head of specimen of Salvelinus alpinus malma, [female], 500 mm. in length from Jaques [sic] Lake.” Bottom: Bajkov’s Figure 3, captioned “Head of specimen of Salvelinus alpinus malma, [male], 590 mm. in length, from Jaques [sic] Lake.” Although only sketches, Bajkov’s figures well illustrate the most characteristic features of this most characteristic Jasper native fish. These are: the terminal mouth cleft bisecting the anterior profile at its midpoint, the elongated head with tapered snout, flat skull, long lower jaw, and eyes placed high on the head (Cavender 1980:300-302; compare with Cavender’s Figure 3). The head structure of bull trout is well suited to an ambush-type predatory style, in which the charr rests on the bottom and watches for prey to pass over. ABSTRACT I conducted an extensive survey of published and unpublished documents to identify the native fish stocks of Jasper National Park, describe their original condition, determine if there is anything unusual or especially significant about them, assess their present condition, outline what is known of their biology and life history, and outline what measures should be taken to manage and protect them. -

Lake Ontario a Voice!
Statue Stories Chicago: The Public Writing Competition Give Lake Ontario a voice! Behind the Art Institute of Chicago, is the Fountain of the Great Lakes. Within the famous fountain is the wistful figure of Lake Ontario. She sits apart from her sister lakes, gazing into the distance with arms outstretched. But what does she have to say for herself? Write a Monologue! Monologos means “speaking alone” in Greek, but we all know that people who speak without thinking about their listener can be very dull indeed. Your challenge is to find a ‘voice’ for your statue and to write an engaging monologue in 350 words. Get under your statue’s skin! Look closely and develop a sense of empathy with the sculpture and imagine how it would feel. How does Lake Ontario feel about her sister lakes? Invite your listener to feel with you: create shifts in tempo and emotion, use different tenses, figures of speech and anecdotes, sensory details and even sound effects. Finding your sculpture’s voice? Write in the first person and adopt the persona of your character: What kind of vocabulary will you use - your own or that of another era/dialect? Your words will be spoken so read them aloud: use their rhythm and your sentence structure to convey emotion and urgency. Read great monologues for inspiration, for example Hamlet’s Alas Poor Yorick, or watch film monologues, like Morgan Freeman’s in The Shawshank Redemption. How will you keep people listening? Structure your monologue! How will you introduce yourself? With a greeting, a warning, a question, an order, a riddle? Grab and hold your listener’s attention from your very first line. -

Indiana Species April 2007
Fishes of Indiana April 2007 The Wildlife Diversity Section (WDS) is responsible for the conservation and management of over 750 species of nongame and endangered wildlife. The list of Indiana's species was compiled by WDS biologists based on accepted taxonomic standards. The list will be periodically reviewed and updated. References used for scientific names are included at the bottom of this list. ORDER FAMILY GENUS SPECIES COMMON NAME STATUS* CLASS CEPHALASPIDOMORPHI Petromyzontiformes Petromyzontidae Ichthyomyzon bdellium Ohio lamprey lampreys Ichthyomyzon castaneus chestnut lamprey Ichthyomyzon fossor northern brook lamprey SE Ichthyomyzon unicuspis silver lamprey Lampetra aepyptera least brook lamprey Lampetra appendix American brook lamprey Petromyzon marinus sea lamprey X CLASS ACTINOPTERYGII Acipenseriformes Acipenseridae Acipenser fulvescens lake sturgeon SE sturgeons Scaphirhynchus platorynchus shovelnose sturgeon Polyodontidae Polyodon spathula paddlefish paddlefishes Lepisosteiformes Lepisosteidae Lepisosteus oculatus spotted gar gars Lepisosteus osseus longnose gar Lepisosteus platostomus shortnose gar Amiiformes Amiidae Amia calva bowfin bowfins Hiodonotiformes Hiodontidae Hiodon alosoides goldeye mooneyes Hiodon tergisus mooneye Anguilliformes Anguillidae Anguilla rostrata American eel freshwater eels Clupeiformes Clupeidae Alosa chrysochloris skipjack herring herrings Alosa pseudoharengus alewife X Dorosoma cepedianum gizzard shad Dorosoma petenense threadfin shad Cypriniformes Cyprinidae Campostoma anomalum central stoneroller -

(Coregonus Lavaretus (L.)) Caused by Competitor Invasion
Speciation Reversal in European Whitefish (Coregonus lavaretus (L.)) Caused by Competitor Invasion Shripathi Bhat1*, Per-Arne Amundsen1, Rune Knudsen1, Karl Øystein Gjelland3, Svein-Erik Fevolden1, Louis Bernatchez2, Kim Præbel1 1 Department of Arctic and Marine Biology, University of Tromsø, Tromsø, Norway, 2 Institut de Biologie Inte´grative et des Syste`mes (IBIS), Universite´ Laval, Que´bec, Canada, 3 Norwegian Institute for Nature Research, Tromsø, Norway Abstract Invasion of exotic species has caused the loss of biodiversity and imparts evolutionary and ecological changes in the introduced systems. In northern Fennoscandia, European whitefish (Coregonus lavaretus (L.)) is a highly polymorphic species displaying adaptive radiations into partially reproductively isolated and thus genetically differentiated sympatric morphs utilizing the planktivorous and benthivorous food niche in many lakes. In 1993, Lake Skrukkebukta was invaded by vendace (Coregonus albula (L.)) which is a zooplanktivorous specialist. The vendace displaced the densely rakered whitefish from its preferred pelagic niche to the benthic habitat harbouring the large sparsely rakered whitefish. In this study, we investigate the potential influence of the vendace invasion on the breakdown of reproductive isolation between the two whitefish morphs. We inferred the genotypic and phenotypic differentiation between the two morphs collected at the arrival (1993) and 15 years after (2008) the vendace invasion using 16 microsatellite loci and gill raker numbers, the most distinctive adaptive phenotypic trait between them. The comparison of gill raker number distributions revealed two modes growing closer over 15 years following the invasion. Bayesian analyses of genotypes revealed that the two genetically distinct whitefish morphs that existed in 1993 had collapsed into a single population in 2008. -

First Record of a Coregonid Fish Species, Coregenus Albula (Linnaeus, 1758) (Salmoniformes: Salmonidae) in Aktaş Lake Shared Between Turkey and Georgia
J. Black Sea/Mediterranean Environment Vol. 25, No. 3: 325-332 (2019) SHORT COMMUNICATION First record of a coregonid fish species, Coregenus albula (Linnaeus, 1758) (Salmoniformes: Salmonidae) in Aktaş Lake shared between Turkey and Georgia Sedat V. Yerli Department of Biology, Hacettepe University, SAL, Beytepe, Ankara, TURKEY Corresponding author: [email protected] Abstract The genus Coregenus (Salmoniformes: Salmonidae) was recently considered not to be represented in Turkey. European cisco or vendace, Coregonus albula (Linnaeus, 1758) was reported for the first time for Turkey in this article with fifteen samples in Aktaş Lake, Ardahan. This species should be added to the checklist of Turkish fish fauna. Turkish name is proposed as “Akbalık” for this species. Keywords: Coregonus albula, first record, Aktaş Lake, Kartsakhi, alkaline lake, Georgia, Turkey Received: 30.10.2019, Accepted: 26.11.2019 Vendace or European cisco Coregonus albula (Linnaeus, 1758) is a native species for northern Europe. Berg (1948) reported the distribution of this species its morphological measurements in the former USSR and adjacent countries. Froese and Pauly (2019) summarized the natural distribution of vendace as Baltic basin, several lakes of upper Volga drainage; some lakes of White Sea basin and North Sea basin east of Elbe drainage; anadromous in Gulf of Finland and marine in northernmost freshened part of Gulf of Bothnia between Finland and Sweden; in Lake Inari, northern Finland; lower Rhine (now extirpated). The vendace was introduced, intentionally in some countries in Europe and United States of America. Vendace was introduced in 1959, 1982-1987 in the Irtysh River Basin and in 1960-61 in Lake Balkhash in Kazakhstan (Mitrofanov and Petr 1999). -
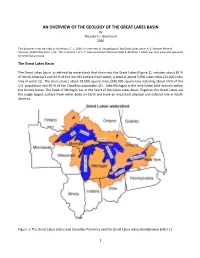
AN OVERVIEW of the GEOLOGY of the GREAT LAKES BASIN by Theodore J
AN OVERVIEW OF THE GEOLOGY OF THE GREAT LAKES BASIN by Theodore J. Bornhorst 2016 This document may be cited as: Bornhorst, T. J., 2016, An overview of the geology of the Great Lakes basin: A. E. Seaman Mineral Museum, Web Publication 1, 8p. This is version 1 of A. E. Seaman Mineral Museum Web Publication 1 which was only internally reviewed for technical accuracy. The Great Lakes Basin The Great Lakes basin, as defined by watersheds that drain into the Great Lakes (Figure 1), includes about 85 % of North America’s and 20 % of the world’s surface fresh water, a total of about 5,500 cubic miles (23,000 cubic km) of water (1). The basin covers about 94,000 square miles (240,000 square km) including about 10 % of the U.S. population and 30 % of the Canadian population (1). Lake Michigan is the only Great Lake entirely within the United States. The State of Michigan lies at the heart of the Great Lakes basin. Together the Great Lakes are the single largest surface fresh water body on Earth and have an important physical and cultural role in North America. Figure 1: The Great Lakes states and Canadian Provinces and the Great Lakes watershed (brown) (after 1). 1 Precambrian Bedrock Geology The bedrock geology of the Great Lakes basin can be subdivided into rocks of Precambrian and Phanerozoic (Figure 2). The Precambrian of the Great Lakes basin is the result of three major episodes with each followed by a long period of erosion (2, 3). Figure 2: Generalized Precambrian bedrock geologic map of the Great Lakes basin. -

Tennessee Fish Species
The Angler’s Guide To TennesseeIncluding Aquatic Nuisance SpeciesFish Published by the Tennessee Wildlife Resources Agency Cover photograph Paul Shaw Graphics Designer Raleigh Holtam Thanks to the TWRA Fisheries Staff for their review and contributions to this publication. Special thanks to those that provided pictures for use in this publication. Partial funding of this publication was provided by a grant from the United States Fish & Wildlife Service through the Aquatic Nuisance Species Task Force. Tennessee Wildlife Resources Agency Authorization No. 328898, 58,500 copies, January, 2012. This public document was promulgated at a cost of $.42 per copy. Equal opportunity to participate in and benefit from programs of the Tennessee Wildlife Resources Agency is available to all persons without regard to their race, color, national origin, sex, age, dis- ability, or military service. TWRA is also an equal opportunity/equal access employer. Questions should be directed to TWRA, Human Resources Office, P.O. Box 40747, Nashville, TN 37204, (615) 781-6594 (TDD 781-6691), or to the U.S. Fish and Wildlife Service, Office for Human Resources, 4401 N. Fairfax Dr., Arlington, VA 22203. Contents Introduction ...............................................................................1 About Fish ..................................................................................2 Black Bass ...................................................................................3 Crappie ........................................................................................7 -

Humber River Watershed Plan Pathways to a Healthy Humber June 2008
HUMBER RIVER WATERSHED PLAN PAThwAYS TO A HEALTHY HUMBER JUNE 2008 Prepared by: Toronto and Region Conservation © Toronto and Region Conservation 2008 ISBN: 978-0-9811107-1-4 www.trca.on.ca 5 Shoreham Drive, Toronto, Ontario M3N 1S4 phone: 416-661-6600 fax: 416-661-6898 HUMBER RIVER WATERSHED PLAN PATHWAYS TO A HEALTHY HUMBER JUNE 2008 Prepared by: Toronto and Region Conservation i Humber River Watershed Plan, 2008 ACKNOWLEDGEMENTS This Humber River Watershed Plan—Pathways to a Healthy Humber—was written by Suzanne Barrett, edited by Dean Young and represents the combined effort of many participants. Appreciation and thanks are extended to Toronto and Region Conservation staff and consultants (listed in Appendix F) for their technical support and input, to government partners for their financial support and input, and to Humber Watershed Alliance members for their advice and input. INCORPORATED 1850 Humber River Watershed Plan, 2008 ii HUMBER RIVER WATERSHED PLAN PATHWAYS TO A HEALTHY HUMBER EXECUTIVE SUMMARY The Humber River watershed is an extraordinary resource. It spans 903 square kilometres, from the headwaters on the Niagara Escarpment and Oak Ridges Moraine down through fertile clay plains to the marshes and river mouth on Lake Ontario. The watershed provides many benefits to the people who live in it. It is a source of drinking water drawn from wells or from Lake Ontario. Unpaved land absorbs water from rain and snowfall to replenish groundwater and streams and reduce the negative impacts of flooding and erosion. Healthy aquatic and terrestrial habitats support diverse communities of plants and animals. Agricultural lands provide local sources of food and green spaces provide recreation opportunities. -

Endangered Species
FEATURE: ENDANGERED SPECIES Conservation Status of Imperiled North American Freshwater and Diadromous Fishes ABSTRACT: This is the third compilation of imperiled (i.e., endangered, threatened, vulnerable) plus extinct freshwater and diadromous fishes of North America prepared by the American Fisheries Society’s Endangered Species Committee. Since the last revision in 1989, imperilment of inland fishes has increased substantially. This list includes 700 extant taxa representing 133 genera and 36 families, a 92% increase over the 364 listed in 1989. The increase reflects the addition of distinct populations, previously non-imperiled fishes, and recently described or discovered taxa. Approximately 39% of described fish species of the continent are imperiled. There are 230 vulnerable, 190 threatened, and 280 endangered extant taxa, and 61 taxa presumed extinct or extirpated from nature. Of those that were imperiled in 1989, most (89%) are the same or worse in conservation status; only 6% have improved in status, and 5% were delisted for various reasons. Habitat degradation and nonindigenous species are the main threats to at-risk fishes, many of which are restricted to small ranges. Documenting the diversity and status of rare fishes is a critical step in identifying and implementing appropriate actions necessary for their protection and management. Howard L. Jelks, Frank McCormick, Stephen J. Walsh, Joseph S. Nelson, Noel M. Burkhead, Steven P. Platania, Salvador Contreras-Balderas, Brady A. Porter, Edmundo Díaz-Pardo, Claude B. Renaud, Dean A. Hendrickson, Juan Jacobo Schmitter-Soto, John Lyons, Eric B. Taylor, and Nicholas E. Mandrak, Melvin L. Warren, Jr. Jelks, Walsh, and Burkhead are research McCormick is a biologist with the biologists with the U.S. -

Nipigon Bay Area of Concern Status of Beneficial Use Impairments September 2010
Nipigon Bay Area of Concern Status of Beneficial Use Impairments September 2010 Nipigon Bay is in the most northerly area of Lake Superior. The Area of Concern takes in a large portion of Nipigon Bay and the Nipigon River, the largest tributary to Lake Superior, and the communities of Red Rock and Nipigon. There are Ontario Power Generation dams on the Nipigon River for the generation of hydroelectricity. The area supports a variety of wetlands and bird populations, including one of four known pelican colonies in Ontario. The watershed forests on both sides of the Nipigon River have been allocated for forest harvesting. Environmental concerns in the Nipigon Bay Area of Concern are related to water level and flow fluctuations in Lake Nipigon and the Nipigon River from the generation of hydroelectricity. These fluctuations affect stream bank stability, sediment load and fish and wildlife habitat. Other concerns include the accumulation of wood fibre, bark and other organic material from past log drives, and effluent discharges from a linerboard mill (which closed in 2006) and the municipal sewage treatment plants in Nipigon and Red Rock. PARTNERSHIPS IN ENVIRONMENTAL PROTECTION Nipigon Bay was designated an Area of Concern in 1987 under the Canada–United States Great Lakes Water Quality Agreement. Areas of Concern are sites on the Great Lakes system where environmental quality is significantly degraded and beneficial uses are impaired. Currently, there are 9 such designated areas on the Canadian side of the Great Lakes, 25 in the United States, and 5 that are shared by both countries. In each Area of Concern, government, community and industry partners are undertaking a coordinated effort to restore environmental quality and beneficial uses through a remedial action plan. -

Ruffe (Gymnocephalus Cernua) Ecological Risk Screening Summary
U.S. Fish and Wildlife Service Ruffe (Gymnocephalus cernua) Ecological Risk Screening Summary US Fish and Wildlife Service, February 2011 Revised, July 2014 Revised, June 2015 Photo: USFWS 1 Native Range, and Status in the United States Native Range From Fuller et al. (2014): “Northern Europe and Asia (Berg 1949; Holcik and Hensel 1974; Wheeler 1978; Page and Burr 1991).” Status in the United States From Fuller et al. (2014): “The ruffe was first identified by Wisconsin DNR in specimens collected from the St. Louis River at the border of Minnesota and Wisconsin in 1987 (Pratt 1988; Pratt et al. 1992; Czypinski et al. 1999, 2000, 2001, 2003). Following that report, reexamination of archived samples revealed misidentified larval specimens of ruffe had been collected from the same area in 1986 (Pratt 1988). The ruffe subsequently spread into Duluth Harbor in Lake Superior and several tributaries of the lake (Underhill 1989; Czypinski et al. 1999, 2000, 2004; Scheidegger, pers. comm.; J. Slade, pers. comm.). It is found in the Amnicon, Flag, Iron, Middle, Raspberry, and Bad rivers, Chequamegon Bay, and Apostle Islands National Lakeshore in Wisconsin (Czypinski et al. 1999, 2000, 2001, 2003, 2004; Tilmant 1999). In August 1994, it was found in Saxon Harbor, Wisconsin, and in the upper peninsula of Michigan at the mouths of the Black and Ontonagon rivers (K. Kindt, pers. comm.). In the lower Peninsula of Michigan along Lake Huron, the first three specimens were caught at the mouth of the Thunder Bay River in August 1995 (K. Kindt, pers. comm.). This species has also been collected in Michigan in Lake Michigan, Lake Superior, Torch Lake, Little Bay de Noc in Escanaba, Big Bay de Noc, Misery River, Ontonagon River, Thunder Bay, and Sturgeon River Sloughs (Czypinski et al. -

The Four Main Groups of the Ojibwe Published on Lessons of Our Land (
The Four Main Groups of the Ojibwe Published on Lessons of Our Land (http://www.lessonsofourland.org) Grades: 6th - 8th Grade Lesson: 1 Unit: 2: American Indian land tenure history Subject: History/Social Studies Additional Subject(s): Geography Achievement Goal: Students will identify the four main groups of the Ojibwe and the large land mass they covered. They will understand that each group lived in a different geographical location and their cultures varied with their environments. Time: One class period Lesson Description: Students learn about the four nations of the Ojibwe tribe and analyze maps of the Great Ojibwe Migration. Teacher Background: Refer to The Four Divisions of the Ojibwe Nation Map found in the Lesson Resources section. This map shows the Ojibwe homeland. It is important to remember that much of it was shared with other tribes. The lines only serve to show where fairly large numbers of Ojibwe lived at one time or another. Within this wide expanse there are great differences in country and climate, and the Ojibwe people adapted their ways of living to their surroundings. In modern times four main groups have been distinguished by location and adaptation to varying conditions. They are the Plains Ojibwe, the Northern Ojibwe, the Southeastern Ojibwe, and the Southwestern Ojibwe or Chippewa. The Plains Ojibwe The Plains Ojibwe live in Saskatchewan, western Manitoba, North Dakota, and Montana. Although they were originally a forest people, they changed their way of life when they moved into the open lands and borrowed many customs of other plains people. Today most of them work at farming and ranching.