Deep Learning with a Recurrent Network Structure in the Sequence Modeling of Imbalanced Data for ECG-Rhythm Classifier
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Automatic Feature Learning Using Recurrent Neural Networks
Predicting purchasing intent: Automatic Feature Learning using Recurrent Neural Networks Humphrey Sheil Omer Rana Ronan Reilly Cardiff University Cardiff University Maynooth University Cardiff, Wales Cardiff, Wales Maynooth, Ireland [email protected] [email protected] [email protected] ABSTRACT influence three out of four major variables that affect profit. Inaddi- We present a neural network for predicting purchasing intent in an tion, merchants increasingly rely on (and pay advertising to) much Ecommerce setting. Our main contribution is to address the signifi- larger third-party portals (for example eBay, Google, Bing, Taobao, cant investment in feature engineering that is usually associated Amazon) to achieve their distribution, so any direct measures the with state-of-the-art methods such as Gradient Boosted Machines. merchant group can use to increase their profit is sorely needed. We use trainable vector spaces to model varied, semi-structured input data comprising categoricals, quantities and unique instances. McKinsey A.T. Kearney Affected by Multi-layer recurrent neural networks capture both session-local shopping intent and dataset-global event dependencies and relationships for user Price management 11.1% 8.2% Yes sessions of any length. An exploration of model design decisions Variable cost 7.8% 5.1% Yes including parameter sharing and skip connections further increase Sales volume 3.3% 3.0% Yes model accuracy. Results on benchmark datasets deliver classifica- Fixed cost 2.3% 2.0% No tion accuracy within 98% of state-of-the-art on one and exceed state-of-the-art on the second without the need for any domain / Table 1: Effect of improving different variables on operating dataset-specific feature engineering on both short and long event profit, from [22]. -

Exploring the Potential of Sparse Coding for Machine Learning
Portland State University PDXScholar Dissertations and Theses Dissertations and Theses 10-19-2020 Exploring the Potential of Sparse Coding for Machine Learning Sheng Yang Lundquist Portland State University Follow this and additional works at: https://pdxscholar.library.pdx.edu/open_access_etds Part of the Applied Mathematics Commons, and the Computer Sciences Commons Let us know how access to this document benefits ou.y Recommended Citation Lundquist, Sheng Yang, "Exploring the Potential of Sparse Coding for Machine Learning" (2020). Dissertations and Theses. Paper 5612. https://doi.org/10.15760/etd.7484 This Dissertation is brought to you for free and open access. It has been accepted for inclusion in Dissertations and Theses by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. Exploring the Potential of Sparse Coding for Machine Learning by Sheng Y. Lundquist A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Computer Science Dissertation Committee: Melanie Mitchell, Chair Feng Liu Bart Massey Garrett Kenyon Bruno Jedynak Portland State University 2020 © 2020 Sheng Y. Lundquist Abstract While deep learning has proven to be successful for various tasks in the field of computer vision, there are several limitations of deep-learning models when com- pared to human performance. Specifically, human vision is largely robust to noise and distortions, whereas deep learning performance tends to be brittle to modifi- cations of test images, including being susceptible to adversarial examples. Addi- tionally, deep-learning methods typically require very large collections of training examples for good performance on a task, whereas humans can learn to perform the same task with a much smaller number of training examples. -

Text-To-Speech Synthesis
Generative Model-Based Text-to-Speech Synthesis Heiga Zen (Google London) February rd, @MIT Outline Generative TTS Generative acoustic models for parametric TTS Hidden Markov models (HMMs) Neural networks Beyond parametric TTS Learned features WaveNet End-to-end Conclusion & future topics Outline Generative TTS Generative acoustic models for parametric TTS Hidden Markov models (HMMs) Neural networks Beyond parametric TTS Learned features WaveNet End-to-end Conclusion & future topics Text-to-speech as sequence-to-sequence mapping Automatic speech recognition (ASR) “Hello my name is Heiga Zen” ! Machine translation (MT) “Hello my name is Heiga Zen” “Ich heiße Heiga Zen” ! Text-to-speech synthesis (TTS) “Hello my name is Heiga Zen” ! Heiga Zen Generative Model-Based Text-to-Speech Synthesis February rd, of Speech production process text (concept) fundamental freq voiced/unvoiced char freq transfer frequency speech transfer characteristics magnitude start--end Sound source fundamental voiced: pulse frequency unvoiced: noise modulation of carrier wave by speech information air flow Heiga Zen Generative Model-Based Text-to-Speech Synthesis February rd, of Typical ow of TTS system TEXT Sentence segmentation Word segmentation Text normalization Text analysis Part-of-speech tagging Pronunciation Speech synthesis Prosody prediction discrete discrete Waveform generation ) NLP Frontend discrete continuous SYNTHESIZED ) SEECH Speech Backend Heiga Zen Generative Model-Based Text-to-Speech Synthesis February rd, of Rule-based, formant synthesis [] -

Unsupervised Speech Representation Learning Using Wavenet Autoencoders Jan Chorowski, Ron J
1 Unsupervised speech representation learning using WaveNet autoencoders Jan Chorowski, Ron J. Weiss, Samy Bengio, Aaron¨ van den Oord Abstract—We consider the task of unsupervised extraction speaker gender and identity, from phonetic content, properties of meaningful latent representations of speech by applying which are consistent with internal representations learned autoencoding neural networks to speech waveforms. The goal by speech recognizers [13], [14]. Such representations are is to learn a representation able to capture high level semantic content from the signal, e.g. phoneme identities, while being desired in several tasks, such as low resource automatic speech invariant to confounding low level details in the signal such as recognition (ASR), where only a small amount of labeled the underlying pitch contour or background noise. Since the training data is available. In such scenario, limited amounts learned representation is tuned to contain only phonetic content, of data may be sufficient to learn an acoustic model on the we resort to using a high capacity WaveNet decoder to infer representation discovered without supervision, but insufficient information discarded by the encoder from previous samples. Moreover, the behavior of autoencoder models depends on the to learn the acoustic model and a data representation in a fully kind of constraint that is applied to the latent representation. supervised manner [15], [16]. We compare three variants: a simple dimensionality reduction We focus on representations learned with autoencoders bottleneck, a Gaussian Variational Autoencoder (VAE), and a applied to raw waveforms and spectrogram features and discrete Vector Quantized VAE (VQ-VAE). We analyze the quality investigate the quality of learned representations on LibriSpeech of learned representations in terms of speaker independence, the ability to predict phonetic content, and the ability to accurately re- [17]. -

Sparse Feature Learning for Deep Belief Networks
Sparse Feature Learning for Deep Belief Networks Marc'Aurelio Ranzato1 Y-Lan Boureau2,1 Yann LeCun1 1 Courant Institute of Mathematical Sciences, New York University 2 INRIA Rocquencourt {ranzato,ylan,[email protected]} Abstract Unsupervised learning algorithms aim to discover the structure hidden in the data, and to learn representations that are more suitable as input to a supervised machine than the raw input. Many unsupervised methods are based on reconstructing the input from the representation, while constraining the representation to have cer- tain desirable properties (e.g. low dimension, sparsity, etc). Others are based on approximating density by stochastically reconstructing the input from the repre- sentation. We describe a novel and efficient algorithm to learn sparse represen- tations, and compare it theoretically and experimentally with a similar machine trained probabilistically, namely a Restricted Boltzmann Machine. We propose a simple criterion to compare and select different unsupervised machines based on the trade-off between the reconstruction error and the information content of the representation. We demonstrate this method by extracting features from a dataset of handwritten numerals, and from a dataset of natural image patches. We show that by stacking multiple levels of such machines and by training sequentially, high-order dependencies between the input observed variables can be captured. 1 Introduction One of the main purposes of unsupervised learning is to produce good representations for data, that can be used for detection, recognition, prediction, or visualization. Good representations eliminate irrelevant variabilities of the input data, while preserving the information that is useful for the ul- timate task. One cause for the recent resurgence of interest in unsupervised learning is the ability to produce deep feature hierarchies by stacking unsupervised modules on top of each other, as pro- posed by Hinton et al. -

Comparison of Feature Learning Methods for Human Activity Recognition Using Wearable Sensors
sensors Article Comparison of Feature Learning Methods for Human Activity Recognition Using Wearable Sensors Frédéric Li 1,*, Kimiaki Shirahama 1 ID , Muhammad Adeel Nisar 1, Lukas Köping 1 and Marcin Grzegorzek 1,2 1 Research Group for Pattern Recognition, University of Siegen, Hölderlinstr 3, 57076 Siegen, Germany; [email protected] (K.S.); [email protected] (M.A.N.); [email protected] (L.K.); [email protected] (M.G.) 2 Department of Knowledge Engineering, University of Economics in Katowice, Bogucicka 3, 40-226 Katowice, Poland * Correspondence: [email protected]; Tel.: +49-271-740-3973 Received: 16 January 2018; Accepted: 22 February 2018; Published: 24 February 2018 Abstract: Getting a good feature representation of data is paramount for Human Activity Recognition (HAR) using wearable sensors. An increasing number of feature learning approaches—in particular deep-learning based—have been proposed to extract an effective feature representation by analyzing large amounts of data. However, getting an objective interpretation of their performances faces two problems: the lack of a baseline evaluation setup, which makes a strict comparison between them impossible, and the insufficiency of implementation details, which can hinder their use. In this paper, we attempt to address both issues: we firstly propose an evaluation framework allowing a rigorous comparison of features extracted by different methods, and use it to carry out extensive experiments with state-of-the-art feature learning approaches. We then provide all the codes and implementation details to make both the reproduction of the results reported in this paper and the re-use of our framework easier for other researchers. -

Convex Multi-Task Feature Learning
Convex Multi-Task Feature Learning Andreas Argyriou1, Theodoros Evgeniou2, and Massimiliano Pontil1 1 Department of Computer Science University College London Gower Street London WC1E 6BT UK [email protected] [email protected] 2 Technology Management and Decision Sciences INSEAD 77300 Fontainebleau France [email protected] Summary. We present a method for learning sparse representations shared across multiple tasks. This method is a generalization of the well-known single- task 1-norm regularization. It is based on a novel non-convex regularizer which controls the number of learned features common across the tasks. We prove that the method is equivalent to solving a convex optimization problem for which there is an iterative algorithm which converges to an optimal solution. The algorithm has a simple interpretation: it alternately performs a super- vised and an unsupervised step, where in the former step it learns task-speci¯c functions and in the latter step it learns common-across-tasks sparse repre- sentations for these functions. We also provide an extension of the algorithm which learns sparse nonlinear representations using kernels. We report exper- iments on simulated and real data sets which demonstrate that the proposed method can both improve the performance relative to learning each task in- dependently and lead to a few learned features common across related tasks. Our algorithm can also be used, as a special case, to simply select { not learn { a few common variables across the tasks3. Key words: Collaborative Filtering, Inductive Transfer, Kernels, Multi- Task Learning, Regularization, Transfer Learning, Vector-Valued Func- tions. -

Visual Word2vec (Vis-W2v): Learning Visually Grounded Word Embeddings Using Abstract Scenes
Visual Word2Vec (vis-w2v): Learning Visually Grounded Word Embeddings Using Abstract Scenes Satwik Kottur1∗ Ramakrishna Vedantam2∗ Jose´ M. F. Moura1 Devi Parikh2 1Carnegie Mellon University 2Virginia Tech 1 2 [email protected],[email protected] {vrama91,parikh}@vt.edu Abstract w2v : farther We propose a model to learn visually grounded word em- eats beddings (vis-w2v) to capture visual notions of semantic stares at relatedness. While word embeddings trained using text have vis-w2v : closer been extremely successful, they cannot uncover notions of eats semantic relatedness implicit in our visual world. For in- stance, although “eats” and “stares at” seem unrelated in stares at text, they share semantics visually. When people are eating Word Embedding something, they also tend to stare at the food. Grounding diverse relations like “eats” and “stares at” into vision re- mains challenging, despite recent progress in vision. We girl girl note that the visual grounding of words depends on seman- eats stares at tics, and not the literal pixels. We thus use abstract scenes ice cream ice cream created from clipart to provide the visual grounding. We find that the embeddings we learn capture fine-grained, vi- sually grounded notions of semantic relatedness. We show Figure 1: We ground text-based word2vec (w2v) embed- improvements over text-only word embeddings (word2vec) dings into vision to capture a complimentary notion of vi- on three tasks: common-sense assertion classification, vi- sual relatedness. Our method (vis-w2v) learns to predict sual paraphrasing and text-based image retrieval. Our code the visual grounding as context for a given word. -

Deep Graphical Feature Learning for the Feature Matching Problem
Deep Graphical Feature Learning for the Feature Matching Problem Zhen Zhang∗ Wee Sun Lee Australian Institute for Machine Learning Department of Computer Science School of Computer Science National University of Singapore The University of Adelaide [email protected] [email protected] Feature Point Coordinate Local Abstract Geometric Feature The feature matching problem is a fundamental problem in various areas of computer vision including image regis- Geometric Feature Net Feature tration, tracking and motion analysis. Rich local represen- Similarity tation is a key part of efficient feature matching methods. However, when the local features are limited to the coor- dinate of key points, it becomes challenging to extract rich Local Feature Point Geometric local representations. Traditional approaches use pairwise Coordinate Feature or higher order handcrafted geometric features to get ro- Figure 1: The graph neural netowrk transforms the coordinates bust matching; this requires solving NP-hard assignment into features of the points, so that a simple inference algorithm problems. In this paper, we address this problem by propos- can successfully do feature matching. ing a graph neural network model to transform coordi- nates of feature points into local features. With our local ference problem in geometric feature matching, previous features, the traditional NP-hard assignment problems are works mainly focus on developing efficient relaxation meth- replaced with a simple assignment problem which can be ods [28, 13, 12, 4, 31, 14, 15, 26, 30, 25, 29]. solved efficiently. Promising results on both synthetic and In this paper, we attack the geometric feature match- real datasets demonstrate the effectiveness of the proposed ing problem from a different direction. -
Unsupervised, Backpropagation-Free Convolutional Neural Networks for Representation Learning
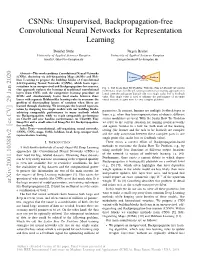
CSNNs: Unsupervised, Backpropagation-free Convolutional Neural Networks for Representation Learning Bonifaz Stuhr Jurgen¨ Brauer University of Applied Sciences Kempten University of Applied Sciences Kempten [email protected] [email protected] Abstract—This work combines Convolutional Neural Networks (CNNs), clustering via Self-Organizing Maps (SOMs) and Heb- Learner Problem bian Learning to propose the building blocks of Convolutional Feedback Value Self-Organizing Neural Networks (CSNNs), which learn repre- sentations in an unsupervised and Backpropagation-free manner. Fig. 1. The Scalar Bow Tie Problem: With this term we describe the current Our approach replaces the learning of traditional convolutional problematic situation in Deep Learning in which most training approaches for layers from CNNs with the competitive learning procedure of neural networks and agents depend only on a single scalar loss or feedback SOMs and simultaneously learns local masks between those value. This single value is used to subsume the performance of an entire layers with separate Hebbian-like learning rules to overcome the neural network or agent even for very complex problems. problem of disentangling factors of variation when filters are learned through clustering. We investigate the learned represen- tation by designing two simple models with our building blocks, parameters. In contrast, humans use multiple feedback types to achieving comparable performance to many methods which use Backpropagation, while we reach comparable performance learn, e.g., when they learn representations of objects, different on Cifar10 and give baseline performances on Cifar100, Tiny sensor modalities are used. With the Scalar Bow Tie Problem ImageNet and a small subset of ImageNet for Backpropagation- we refer to the current situation for training neural networks free methods. -

Sparse Estimation and Dictionary Learning (For Biostatistics?)
Sparse Estimation and Dictionary Learning (for Biostatistics?) Julien Mairal Biostatistics Seminar, UC Berkeley Julien Mairal Sparse Estimation and Dictionary Learning Methods 1/69 What this talk is about? Why sparsity, what for and how? Feature learning / clustering / sparse PCA; Machine learning: selecting relevant features; Signal and image processing: restoration, reconstruction; Biostatistics: you tell me. Julien Mairal Sparse Estimation and Dictionary Learning Methods 2/69 Part I: Sparse Estimation Julien Mairal Sparse Estimation and Dictionary Learning Methods 3/69 Sparse Linear Model: Machine Learning Point of View i i n i Rp Let (y , x )i=1 be a training set, where the vectors x are in and are called features. The scalars y i are in 1, +1 for binary classification problems. {− } R for regression problems. We assume there is a relation y w⊤x, and solve ≈ n 1 min ℓ(y i , w⊤xi ) + λψ(w) . w∈Rp n Xi=1 regularization empirical risk | {z } | {z } Julien Mairal Sparse Estimation and Dictionary Learning Methods 4/69 Sparse Linear Models: Machine Learning Point of View A few examples: n 1 1 i ⊤ i 2 2 Ridge regression: min (y w x ) + λ w 2. w∈Rp n 2 − k k Xi=1 n 1 i ⊤ i 2 Linear SVM: min max(0, 1 y w x ) + λ w 2. w∈Rp n − k k Xi=1 n 1 i i −y w⊤x 2 Logistic regression: min log 1+ e + λ w 2. w∈Rp n k k Xi=1 Julien Mairal Sparse Estimation and Dictionary Learning Methods 5/69 Sparse Linear Models: Machine Learning Point of View A few examples: n 1 1 i ⊤ i 2 2 Ridge regression: min (y w x ) + λ w 2. -

Self-Supervised Feature Learning by Learning to Spot Artifacts
Self-Supervised Feature Learning by Learning to Spot Artifacts Simon Jenni Paolo Favaro University of Bern, Switzerland {jenni,favaro}@inf.unibe.ch Abstract We introduce a novel self-supervised learning method based on adversarial training. Our objective is to train a discriminator network to distinguish real images from im- ages with synthetic artifacts, and then to extract features from its intermediate layers that can be transferred to other data domains and tasks. To generate images with artifacts, we pre-train a high-capacity autoencoder and then we use a damage and repair strategy: First, we freeze the autoen- coder and damage the output of the encoder by randomly dropping its entries. Second, we augment the decoder with a repair network, and train it in an adversarial manner Figure 1. A mixture of real images (green border) and images with against the discriminator. The repair network helps gen- synthetic artifacts (red border). Is a good object representation erate more realistic images by inpainting the dropped fea- necessary to tell them apart? ture entries. To make the discriminator focus on the arti- Towards this goal, we propose to learn features by clas- facts, we also make it predict what entries in the feature sifying images as real or with artifacts (see Figure 1). We were dropped. We demonstrate experimentally that features aim at creating image artifacts, such that a model capable learned by creating and spotting artifacts achieve state of of spotting them would require an accurate representation the art performance in several benchmarks. of objects and thus build features that could transfer well to tasks such as object classification, detection and segmenta- tion.