Effect of Georgetown Lake on the Water Quality of Clear Creek, Georgetown, Colorado, 1997Ð98
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
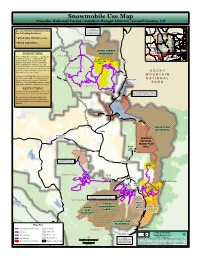
Snowmobile Use Map for Sulphur Ranger District
Snowmobile Use Map Arapaho National Forest - Sulphur Ranger District - Grand County, CO Check with Groomed Routes Maintained by Parks Ranger District JACKSON Routt National Forest LARIMER the Following Partners: UV750 for snowmobile information * Grand Lake Trail Groomers ¤£40 ¤£34 GRAND * Grand Adventures Granby BOULDER AREA 40 OF ¤£ GILPIN Denver NEVER SUMMER DETAIL PROHIBITIONS WILDERNESS EAGLE CLEAR ¨¦§70 A R A P A H O CREEK It is prohibited to possess or operate an UV123 SUMMIT over-snow vehicle on National Forest 0 10 20 B O W E N G U L C H Sulphur Ranger District Miles System lands on the Sulphur Ranger B O W E N G U L C H P R O T E C T II O N District other than in accordance with 34 A R E A ¤£ these designations (CFR 261.14). An over-snow vehicle is a motor vehicle that «¬125 is designated for use over snow and that N A T II O N A L runs on a track or tracks and/or a ski or skis while in use over snow. R O C K Y +$491 Violations of 36 CFR 261.14 are subject M O U N T A I N to a fine of up to $5,000, imprisonment 108 N A T I O N A L for up to 6 months, or both (18 U.S.C. UV Grand Lake Gr an 3571(e)). This prohibition applies regard- d s La P A R K e ke R less of the presence or absence of signs. -

Historical Mine Report Files C. 1900 - 1980 at the Colorado State Archives
Historical Mine Report Files c. 1900 - 1980 at The Colorado State Archives "C" Mine County Notes C - CM - 25 Montrose Miscellaneous "C" C - G - 26 Mesa C - JD - 6 Montrose Miscellaneous "C" C - SR - 10 San Miguel C - SR - 16 A San Miguel C & C Drilling San Miguel Miscellaneous "C" C & F La Plata C & H Mining Company San Miguel Miscellaneous "C" C & K Drilling Company San Miguel Miscellaneous "C" C- JD - 5 Montrose Miscellaneous "B" C. B. Clear Creek Miscellaneous "C" C. F. & I Water Tunnel Fremont Miscellaneous C C. F. C. & Moonbeam Montrose C. H. Mining Company Ouray Miscellaneous "C" C. J. Dold Mining Lake C. K. & N. Mine Teller C. O. D. Mine Teller C. P. Molybdenum Corporation Mesa Miscellaneous "C" C. R. Conover & Brothers Chaffee Miscellaneous "C" C. W. A. Project No. 503 - Rock for Platte River Rip-Rap Jefferson Miscellaneous C C-a Tract Rio Blanco Cabin Creek Clear Creek Cabinet Group Summit Cabinsite Matchless No. 10 Gilpin Caburg Mining Company Hinsdale Miscellaneous C Caddo Mining Clear Creek Caddoa Reservoir Project Bent Miscellaneous "C" Cadiz Mining Gilpin Cady Mines Company Lake Miscellaneous C Calamine Mine San Miguel Miscellaneous "C" Calamity Creek Mesa Calamity Group Mesa Calco Mesa Caldona Mine Gilpin Miscellaneous "C" Caldora Mine Park Caledonia Teller Caledonia Mine Boulder Miscellaneous "C" Caledonia Mine San Juan Caledonia No. 2 Gilpin Calhoun Mine Gilpin California Dolores California Saguache California - Hidden Treasure Gilpin California Gulch Shaft Lake California Mine Boulder California Mine Hinsdale Miscellaneous C Callahan Holdings Garfield Calliope Mine Ouray Calmett Tunnel at Ilse Custer Miscellaneous "C" Calumet No. -

A TIMELINE for GOLDEN, COLORADO (Revised October 2003)
A TIMELINE FOR GOLDEN, COLORADO (Revised October 2003) "When a society or a civilization perishes, one condition can always be found. They forgot where they came from." Carl Sandburg This time-line was originally created by the Golden Historic Preservation Board for the 1995 Golden community meetings concerning growth. It is intended to illustrate some of the events and thoughts that helped shape Golden. Major historical events and common day-to-day happenings that influenced the lives of the people of Golden are included. Corrections, additions, and suggestions are welcome and may be relayed to either the Historic Preservation Board or the Planning Department at 384-8097. The information concerning events in Golden was gathered from a variety of sources. Among those used were: • The Colorado Transcript • The Golden Transcript • The Rocky Mountain News • The Denver Post State of Colorado Web pages, in particular the Colorado State Archives The League of Women Voters annual reports Golden, The 19th Century: A Colorado Chronicle. Lorraine Wagenbach and Jo Ann Thistlewood. Harbinger House, Littleton, 1987 The Shining Mountains. Georgina Brown. B & B Printers, Gunnison. 1976 The 1989 Survey of Historic Buildings in Downtown Golden. R. Laurie Simmons and Christine Whitacre, Front Range Research Associates, Inc. Report on file at the City of Golden Planning and Development Department. Survey of Golden Historic Buildings. by R. Laurie Simmons and Christine Whitacre, Front Range Research Associates, Inc. Report on file at the City of Golden Planning and Development Department. Golden Survey of Historic Buildings, 1991. R. Laurie Simmons and Thomas H. Simmons. Front Range Research Associates, Inc. -

Chapter 4-8 Water Resources & Water Quality
TABLE OF CONTENTS Page 4.8 WATER RESOURCES AND WATER QUALITY .............................................................................4.8-1 4.8.1 Affected Environment....................................................................................................4.8-1 4.8.1.1 Surface Water ......................................................................................................... 4.8-1 4.8.1.2 Groundwater .......................................................................................................... 4.8-8 4.8.1.3 Water Quality/Uses............................................................................................. 4.8-11 4.8.1.4 Drinking Water Sources ..................................................................................... 4.8-13 4.8.1.5 Non-Potable Water Supplies ............................................................................. 4.8-14 4.8.1.6 Drainage System .................................................................................................. 4.8-14 4.8.1.7 Characterization of Typical Roadway Runoff................................................. 4.8-14 4.8.2 Environmental Consequences .................................................................................... 4.8-15 4.8.2.1 Water Quality ....................................................................................................... 4.8-15 4.8.2.2 Impacts Common to All Build Alternatives.................................................... 4.8-20 4.8.2.3 No Action Alternative ....................................................................................... -
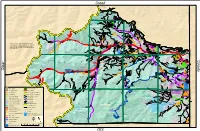
C Lear Creek GIS C Ounty
Creek G ilpin C o unty D D D 12147 D Jefferson County G ty Gilpin County rand Coun ICE LAKE D OHMAN LAKE STEUART LAKE D D REYNOLDS LAKE D D 13391 LAKE CAROLINELOCH LOMAND ST MARYS GLACIER Fox Mountain ST MARYS DLAKE FALL RIVER SILVER LAKE D D LAKE QUIVIRA 11239 13130 FALL RIVER RESERVOIR SLATER LAKE D SILVER CREEK SHERWIN LAKECHINNS LAKE Witter Peak D D 12884 D D James Peak Wilderness MEXICAN GULCH D ETHEL LAKEBYRON LAKE D D BILL MOORE LAKE HAMLIN GULCH D D 13132 CUMBERLAND GULCH D MILL CREEK D D Russell Peak Breckinridge Peak Berthoud Pass D D 12889 G D D ilp D in D C D ou n ty D Grand C D D ounty D MAD CREEK LION CREEK D Stanley Mountain YORK GULCH D D FALL RIVER 12521 BLUE CREEK Cone Mountain D D HOOP CREEK 12244 SPRING GULCH Red Elephant Hill D 10316 D ¤£US 40 D CLEAR CREEK This map is visual representation only, do not use Bellevue Mountain URAD RESERVOIR (LOWER) for legal purposes. Map is not survey accurate and ¨¦§I 70 D WEST FORK CLEAR CREEK 9863 Seaton Mountain may not comply with National Mapping Accuracy Red Mountain D D GUANELLA RESERVOIR 9105 12315 EMPIRE n County Standards. Map is based on best available data as Gilpi RUBY CREEK Ball Mountain Douglas Mountain of October, 2018 . BUTLER GULCH CENTRAL CITY D D VIRGINIA CANYON Lincoln Mountain GEORGIA GULCH 12529 9550 OHIO GULCH WOODS CREEK D GILSON GULCH Engelmann Peak 10363 TURKEY GULCH D IDAHO HASSELL LAKEURAD RESERVOIR (UPPER) 13362 BARD CREEK LAKE SILVER CREEK TRAIL CREEK J e US 6 f f ¤£ e r s o BARD CREEK SPRINGS n Flirtation Peak C Robeson Peak Columbia Mountain o ty -

Georgetown–Silver Plume Historic District
Teacher Resource Set Title Georgetown-Silver Plume Historic District Developed by Cathy Fuller, Middle School Teacher, Guardian Angels School, Denver CO Grade Level 4 Essential Question Who were the early settlers of Georgetown and Silver Plume? How did social class determine the development of each of these towns? And how did this influence the architecture and design of each? How has this region been able to prosper over time? Contextual Paragraph According to the Colorado Encyclopedia, the Georgetown-Silver Plume Historic District is one of the best-preserved historic mining districts in Colorado. Located in the upper Clear Creek Valley, Georgetown thrived as a commercial and professional center, while Silver Plume developed as a diverse town of working-class miners. The diversities in these two towns provides an excellent opportunity to understand the differences in the cultures tied to the practice in mining throughout Colorado. Silver Plume, home to the men who worked in the mines, is a hodgepodge of different ethnicities. The different sections in the Silver Plume cemetery (organized by religion and ethnicity) offer a greater understanding of who lived and worked in the region. Similarly, the architecture of homes and other structures in Georgetown speak to the class of those who lived in the town. Significant architectural-style structures found in the Historic District and described in the National Register of Historic Places include Hamill House, Maxwell House, Luedde House, Spencer House, Bowman-White House, Public School, Hotel de Paris, Clear Creek County Court House, the Old Jail and Grace Episcopal Church. After World War II, this area evolved into a tourist attraction and continues to be a popular destination for Front Range residents. -

Clear Creek Ranger Distric MVUM
United States Department of Agriculture of Department States United 420000 430000 440000 450000 460000 470000 Forest Service Forest 105°52'30"W 105°45'0"W 105°37'30"W 105°30'0"W 105°22'30"W Legend +$ +$ Thorodin Mtn Colorado JAN-01-2010 Colorado ) Roads Open to Highway Legal Vehicles PURPOSE AND CONTENTS Roads Open to All Vehicles Seasonal Designation (See Table) America's Great Outdoors Great America's 39°52'30"N OF THIS MAP ! ! ! Dispersed Camping Golden Gate Canyon ! ! ! (See Dispersed Camping Table) State Park ! The designations shown on this motor vehicle use map ! 39°52'30"N ! are effective as of the date on the front cover and will Interstate ! ! 719.1C ! ! ! ! ! remain in effect until superseded by the next year's motor ! Highways, US, State ! 4N ! ! ! ! 7! ! 1 ! 9 ! . ! 1 ! +$ ! ! ! ! ! ! vehicle use map. It is the responsibility of the user to ! ! ! 7 ! ) ! Other Public Roads 1 ! ! ! 6 ! Dakota Hill ! acquire the current MVUM.This map shows the National ! ! ! . 1 70333 ! ! ! ! ! t ! ! ! 1 ! ! ! ! c ! . Short Route Identifier i ! ! r ! 3 t ! ! Forest System roads, National Forest System trails, and ! ! 4 s ! ! Apex i ! ! ! 71 ! D ! $ t ! + ! r ! s ! ! ! ! # ! G e e ! ! the areas on National Forest System lands in the Clear Milepost Marker g r ! n o ! ! ! ! ! a F ! i R l ! l a ! G r n ! p e ! Creek Ranger District that are designated for motor vehicle ! d io 1 FS Campground l t . ! i u a ! 9 r 7 n o N ! ! ! use pursuant to 36 Code of Federal Regulations (CFR) a B lt 7 1 7 e 5 ! ! ! n C v 3 ! e . -

Empire Junction
MP 253 – 254: The Big Chief Overlook and “the Glen” The mile between MP 253 and 254 has lots of history in it. It includes the only recorded remains of the Victory Highway on the Clear Creek County side of the Pass, an overlook where a Castle once stood and one of the author’s favorite places on Berthoud Pass. Route Mile Marker X Y Longitude Latitude 1U. S. Highway 40 253 436421.26 4401861.97 -105.742283 39.764310 254 438019.73 4401757.05 -105.723612 39.763483 The road between these two mileage posts has always been part of the original auto road. Sections of the original wagon road can be seen below in the valley and are used today as Morrison Road. The top two photos show the road as it travels west out of Empire and begins to climb up Berthoud Pass. Sections of the original wagon road were used for the footprint of the auto road in Mileage Posts 252-253 and also in 254 – 255, but not this one. The bottom photo is in the same area but is taken looking east toward Empire. The top photo was provided by the Federal Highway Administration. The middle photo is provided by the Historical Society of Idaho Springs. The bottom photo is provided by and used with permission from the Denver Public Library. 1 Taken from the Colorado Department of Transportation’s website: http://apps.coloradodot.info/dataaccess/Highways/index.cfm?fuseaction=HighwaysMain MP 253 – 254: Big Chief Overlook and “the Glen” (PAW 2012) 1 Between these two mileage posts is a large pull-out on the left (or south) side of the road as you are going west from Empire. -

Clear Creek/Central City Explanation of Significant
SDMS Document ID -.-. A Strn o fnn!»*o* 2008929 CLEAR CREEK/CENTRAL CITY SUPERFUND SITE EXPLANATION OF SIGNIFICANT DIFFERENCES ARGO & BIG FIVE MINE WASTE PILES INTRODUCTION The Clear Creek/Central City Superfund Site (the Site) is located on the east slope of Colorado's Front Range, approximately 30 miles west of Denver (Figure 1). The Site was placed on the National Priorities List in September 1983 because of impacts to Clear Creek from historic mining activities. EPA and the Colorado Department of Public Health and the Environment (CDPHE) refer to the Clear Creek basin as the Clear Creek/Central City Superfund Study Area. Within this broad study area, several discrete draining mines and mine dumps have been identified as the Site. Currently included in the Site are 23 properties - six mine tunnels and 17 mine waste piles. The Argo and Big Five mine waste piles are two of these properties and are the subject of this document. They are both located in Idaho Springs, Colorado (Figure 2). EPA selected a remedy for the Argo and Big Five mine waste piles in the Operable Unit #2 Record of Decision (OU#2 ROD) which was signed March 31, 1988. This document explains the significant difference between the remedy selected in the OU#2 ROD and the one now planned for the Argo and Big Five mine waste piles. CDPHE is the lead agency for the Site and is conducting the remedial design and remedial action for these properties with funds provided by EPA via cooperative agreements. Under Section 117 of the Comprehensive Environmental Response, Compensation, and Liability Act of 1980 (CERCLA or Superfund), as amended by the Superfund Amendments and Reauthorization Act of 1986 (SARA), 42 U.S.C. -
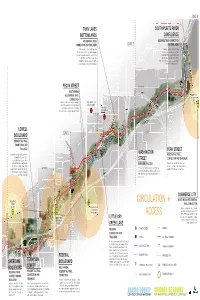
Maps, Basic Needs, and Guidelines.Pdf
ZONE 3 TWIN LAKES SOUTH PLATTE RIVER BOTTOMLANDS CONFLUENCE RESIDENTIAL TRAIL REGIONAL TRAIL CONNECTION CONNECTION AND TRAILHEAD ZONE 2 AND TRAILHEAD Trail connections from Scott Carpenter Parking, restroom and pedestrian Middle School and Valley View Elementary improvements recommended. Future open up new outdoor learning environments development should expand pedestrian in the Twin Lakes Bottomlands. New and bicycle access from South Platte walking trails provide access to wildlife River and Commerce City Commuter Rail. areas while preserving wildlife habitats. Preferred bike share location. T S PLATTE RIVER E 73rd AVE YORK STREET PECOS STREET CONFLUENCE MULTI-MODAL US RESIDENTIAL TRAIL 270 COMMERCE CITY STATION CONNECTION AREA Improved street and underpass crossings, SCOTT CARPENTER pedestrian connection and neighborhood MIDDLE SCHOOL trail signage for Pecos Street Station, VALLEYVIEW Berkley/Zuni and Sunnyside neighborhoods. ELEMENTARY US STREET WASHINGTON 25 W 70th AVE T T TWIN LAKES US T PARK 76 LOWELL BROADWAY T E 68th AVE BOULEVARD ZONE 1 T L RESIDENTIAL TRAIL ITT LE D TWINT N LAKES KE CONNECTION AND R Y BBOTTOMLANDS C TRAILHEAD R E YORK STREET E K WASHINGTON Complete multi-use trail connections T RESIDENTIAL TRAIL R T to Jim Baker Reservoir, Clear A T I L CONNECTION AND TRAILHEAD Creek Valley Park, Lowell Ponds STREET and Carl Park Community Center. EMERGENCY ACCESS Planned improvements include a new Neighborhood trail signage helps T T T sidewalk and trail connection. Additional navigation from Westminster, Arvada W 64th AVE New access for Emergency Services. Potential neighborhood signage recommended. and Aloha Beach, and from Berkeley connections to planned detached walk. Future development should seek to provide additional and Regis University. -

THE COLORADO MAGAZINE Published Bi-Monthly by the State Historical Society of Colorado
THE COLORADO MAGAZINE Published bi-monthly by The State Historical Society of Colorado Vol. XVII Denver, Colorado, May, 1940 No. 3 Place Names in Colorado (B)* Bachelor, Mineral County. Named for the Bachelor Mine, which was staked out in 1884 by George 'Wilson. One version is that the camp was so-named because of the absence of women. Between 1895 and 1908 the town had a population of more than a thousand inhabitants. It was abandoned after the highway was built, the population moving to Jim Town, which later became Creede. 1 Bachelor Switch, Ouray County. This town was named for the Bachelor Mine, Uncompahgre District,2 and its location at a switch on the Rio Grande Southern Railroacl. 3 Bailey, Park County. Bailey was named for a settler, William Bailey, who established a hotel and stage station here in 1864. 'l'he station was known as Bailey's Ranch; the later settlement adopted the name, later shortening it.4 In 1878 the narrow-gage Denver & South Park Railroad made Bailey its terminal. Bakers Crossing, Arapahoe County. In 1859 Jim Baker, fa. mous Mountain Man, established a small store here and operated a toll bridge, built to replace the old ferry ; hence the name. Baker's adobe house stood on the south side of Clear Creek, near what is now 53rd and 'l'ennyson Streets in the present city of Denver.5 Bakers Park, San Juan County. This was the first permanent settlement on the present site of Silverton, and was named for the surrounding area, which had been called Bakers Park since 1860. -

Colorado Clear Creek Located on 1-70 Right-Of-Way
Form 10-300 UNITED STATES DEPARTMENT OF THE INTERIOR (July 1969) NATIONAL PARK SERVICE Colorado NATIONAL REGISTER OF HISTORIC PLACES Clear Creek INVENTORY - NOMINATION FORM FOR NPS USE ONLY ENTRY NUMBER A/E/ (Type all entries — complete applicable sections) COMMON: Silver Plume Depot AND/OR HISTORIC: same STREET AND NUMBER: Located on 1-70 right-of-way, adjacent to east-bound highway CITY OR TOWN: entrance ramp at Silver Plume STATE COUNTY: Colorado 08 Clear Creek 019 CATEGORY ACCESSIBLE OWNERSHIP STATUS ( Check One) TO THE PUBLIC District gg Building §£l p u b '' c Public Acquisition: (2£| Occupied Yes: 0 Restricted Site Q Structure D Private || In Process [~1 Unoccupied Q] Unrestricted Q Object D Botrl ( | Being Considered Q Preservation work in progress D No PRESENT USE (Check One or More as Appropriate) I | Agricultural | | Government | | Park f~] Transported on Comments Q Commercial CD Industrial Q Private Residence {$£1 Other (Specify) ___________. O Educational CD Military Q Religious Leased by the Loveland [ | Entertainment 1~1 Museum | | Scientific Patrol Associates, Inc. OWNER'S NAME: ?T O State Historical Society of Colo. (depot) ; State Highway Dept r-> STREET AND NUMBER: (land) l-{O p 200 Fourteenth Avenue a CITY OR TOWN: o Denver Colorado 08 ti$iiijil:;ili:iiiiilislriii!ii;- COURTHOUSE, REGISTRY OF DEEDS, ETC: Clear Creek County Courthouse 0 STREET AND NUMBER: CITY OR TOWN: Georgetown Colorado 08 TITLE OF SURVEY:'• State Highway No.91 Clear Creek County-Federal Aid Project No. 1-70 (original location - proposed relocation) DATE OF SURVEY: 1^66 D Federal j(f| State County Local DEPOSITORY FOR SURVEY RECORDS: State Museum STREET AND NUMBER: 200 Fourteenth Avenue O CITY OR TOWN: STATE: Denver Colorado Ob5 - *4r (Check One) Excellent Q Good Fair Q Deteriorated Q Ruins Q Unexposed CONDITION (Check One) (Check One) Altered D Unaltered Moved Q Original Site DESCRIBE THE PRESENT AND ORIGINAL (if known) PHYSICAL APPEARANCE The Silver Plume Depot is constructed upon a wood^frame, with vertical board and batten exterior walls, off-slilf~at ceiling level.