Modelling the Population Dynamics of the Mt. Graham Red Squirrel: Can We Predict Its Future in a Changing Environment with Multiple Threats?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Birds of Coniferous Forest on Mount Graham, Arizona
Wilson Bull., 107(4), 1995, pp. 719-723 SHORT COMMUNICATIONS Birds of coniferous forest on Mount Graham, Arizona.-Because of interest in the effect upon the biota of Mount Graham by current development for astronomy, I repeated observations and censuses made there forty years ago. Avifaunal changes have occurred. Unlike my companion study in the Sierra Nevada (Marshall 1988), the losses at Mount Graham of nesting species-all at the lower altitudinal limit of Pinus Zeiophylla chihuahuana and P. ponderosa arizonica-lack an obvious connection to human interference with the environment. Study area.-Mount Graham, in southeastern Arizona, is capped with old growth Engel- mann spruce and alpine fir from 3000 m to the summit at 3267 m. This boreal forest of 8 km* is at its southernmost limits on the North American continent. Descending, one passes through other vegetation zones in sequence: Douglas firs and white firs mixed with spruce, and then mixed with Mexican white pines; south slopes of New Mexican locust and Gambel oak; ponderosa pine with Gambel oak, ponderosa pine mixed with silver-leaf oak; and finally Chihuahua pine with Arizona oak (Martin and Fletcher 1943, Hoffmeister 1956, Marshall 1957, Mohlenbrock 1987). From memory, notes, and photographs I detect no change in vegetation during the forty-year study period. Specifically, the trees at the mapped census area in Wet Canyon (Fig. 1) have not closed ranks about the little openings suitable for those foothill birds that forage among grasses, boulders, manzanitas, and nolinas. The mesic luxuriance of Mount Graham’s vegetation is shown by running streams sup- porting tall groves of maples, by the enormous Douglas firs that remain, by the profusion of understory flowers and green forbs, and by the gigantic stature of clear-trunked alders dominating Wet Canyon. -

236 Pinaleño Mountains in the Twentieth Century Atalanta Hoyt
Pinaleño Mountains in the Twentieth Century Atalanta Hoyt Throughout the twentieth century, a few major events dominated the history of the Forest Service. First, the founding of the National Forest Service in 1905 replaced the Bureau of Forestry and led to the creation of modern National Forests. The new service was created under the jurisdiction of the Department of Agriculture with the purpose of securing a long term supply of timber for the American people.1 Second, the great depression of the 1930s, Franklin Roosevelt’s creation of the Civilian Conservation Corps (CCC) and the expansion of the Forest Service changed the shape of National Forests.2 This time period featured a major transition from timber management to hands on putting resources into the forest. The Forest Service and CCC planted trees, carved trails, built roads, and conducted research; actively molding forests and applying the latest forestry techniques instead of letting the forest take its course.3 A third period of great change came in the 1970s during the environmental era.4 The emphasis changed from conceptualizing the forests as resources to be converted into marketable goods to seeing them as wilderness in need of preservation. While conservation has always been an important part of the Forest Service - advocated by both those who saw an intrinsic value in wilderness and by those who used the wilderness for recreational purposes - increased urbanization highlighted the uniqueness of forests. Efforts to catalog and protect the environments of forests became a main priority while ecologists and conservationists gained status.5 These three main shifts defined the Forest Service in the twentieth century. -

Relational Database Systems 1
Relational Database Systems 1 Wolf-Tilo Balke Jan-Christoph Kalo Institut für Informationssysteme Technische Universität Braunschweig www.ifis.cs.tu-bs.de Summary last week • Data models define the structural constrains and possible manipulations of data – Examples of Data Models: • Relational Model, Network Model, Object Model, etc. – Instances of data models are called schemas • Careful: Often, sloppy language is used where people call a schema also a model • We have three types of schemas: – Conceptual Schemas – Logical Schemas – Physical Schemas • We can use ER modeling for conceptual and logical schemas Relational Database Systems 1 – Wolf-Tilo Balke – Institut für Informationssysteme – TU Braunschweig 2 Summary last week • Entity Type Name • Weak Entity Type Name • Attribute name • Key Attribute name • name Multi-valued Attribute name name • Composite Attribute name • Derived Attribute name • Relationship Type name • Identifying Relationship Type name EN 3.5 Relational Database Systems 1 – Wolf-Tilo Balke – Institut für Informationssysteme – TU Braunschweig 3 Summary last week • Total participation of E2 in R E1 r E2 • Cardinality – an instance of E1 may relate to multiple instances of E2 (0,*) (1,1) E1 r E2 • Specific cardinality with min and max – an instance of E1 may relate to multiple instances of E2 (0,*) (0,1) E1 r E2 EN 3.5 Relational Database Systems 1 – Wolf-Tilo Balke – Institut für Informationssysteme – TU Braunschweig 4 3 Extended Data Modeling • Alternative ER Notations • Extended ER – Inheritance – Complex Relationships -

Summits on the Air – ARM for the USA (W7A
Summits on the Air – ARM for the U.S.A (W7A - Arizona) Summits on the Air U.S.A. (W7A - Arizona) Association Reference Manual Document Reference S53.1 Issue number 5.0 Date of issue 31-October 2020 Participation start date 01-Aug 2010 Authorized Date: 31-October 2020 Association Manager Pete Scola, WA7JTM Summits-on-the-Air an original concept by G3WGV and developed with G3CWI Notice “Summits on the Air” SOTA and the SOTA logo are trademarks of the Programme. This document is copyright of the Programme. All other trademarks and copyrights referenced herein are acknowledged. Document S53.1 Page 1 of 15 Summits on the Air – ARM for the U.S.A (W7A - Arizona) TABLE OF CONTENTS CHANGE CONTROL....................................................................................................................................... 3 DISCLAIMER................................................................................................................................................. 4 1 ASSOCIATION REFERENCE DATA ........................................................................................................... 5 1.1 Program Derivation ...................................................................................................................................................................................... 6 1.2 General Information ..................................................................................................................................................................................... 6 1.3 Final Ascent -

Arizona IGHWAYJ:~~-= E.Mber · · · - Narrow and Unsafe Bridges
ARIZOnA IGHWAYJ:~~-= E.mBER · · · - Narrow and Unsafe Bridges A Lasting Monument . TO A GREATENGINEER Are Quickly, Economically and Lastingly Replaced With UILDER, executive, soldier, friend - Tom O'Connell has joined that silent host of Billustrious engi neers whose achievements are their end uring monuments. Armco Multi Plate Waterways His death on November 3, 1937, mark ed the end of a career of distinguished service Full Circles 01· Arches, Singly or in Battery, they care for streams up to his state and country ·that covered a period of almost a quarter of .a century. Time, to several hundred square feet in cross sectional area. Write for Illus immutable yardstick of values, will list his name conspiciously in the roll of those who trated Literature. built America's great Southwest. His works will long live af ter him. Western Metal ManufacturingCompany 1500 South Central Avenue P. 0. Box 1585 Phoenix, Arizona DECEMBER,1937 ARIZONA HIGHWAYS 3 The courtesy of the open range was are many denominational hospital s and Tucson numbers some of the most pr om th e rule most of th e time, except when sanitoriums, private sanitoriums, rest inent people, and man y of them come the Apaches went on a rampage, slaying, homes and "rest ranches," where privacy just for th e rod eo. Tucs on is also host pillaging and running off all available and specialized supervision are ava ilable. to many persons of international fam e. livestock. A welcome to strangers One of the most outstanding of these Each year it is becoming more and mor e meant offering the best. -

Mount Graham Red Squirrel Recovery Plan
MOUNT GRAHAM RED SQUIRREL Tamiasciurus hudsonicus arahamensie RECOVERY PLAN Prepared by Lesley A. Fitzpatrick, Member, Recovery Team U.S. Fish and Wildlife Service Phoenix, Arizona Genice F. Froehlich, Consultant, Recovery Team U.S.D.A. Forest Service Coronado National Forest Safford, Arizona Terry B. Johnson, Member, Recovery Team Arizona Game and Fish Department Phoenix, Arizona Randall A. Smith, Leader, Mt. Graham Red Squirrel Recovery Team U.S.D.A. Forest Service Coronado National Forest Tucson, Arizona R. Barry Spicer Arizona Game and Fish Department Phoenix, Arizona for Region 2 U.S. Fish and Wildlife Service Albuquerque, New Mexico Date: Disclaimer Page Recovery plans delineate reasonable actions which are believed to be required to recover and/or protect the species. Plans are prepared by the U.S. Fish and Wildlife Service, sometimes with the assistance of recovery teams, contractors, State agencies, and others. Objectives will only be attained and funds expended contingent upon appropriations, priorities, and other budgetary constraints. Recovery plans do not necessarily represent the views nor the official positions or approvals of any individuals or agencies, other than the U.S. Fish and Wildlife Service, involved in the plan formulation. They represent the official position of the U.S. Fish and Wildlife Service & after they have been signed by the Regional Director as aDproved. Approved recovery plans are subject to modification as dictated by new findings, changes in species status, and the completion of recovery tasks. Literature citations should read as follows: U.S. Fish and Wildlife Service. 1992. Mount Graham Red Squirrel Recovery Plan. U.S. Fish and Wildlife Service, Albuquerque, New Mexico. -
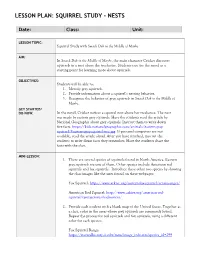
Lesson Plan: Squirrel Study - Nests
LESSON PLAN: SQUIRREL STUDY - NESTS Date: Class: Unit: LESSON TOPIC: Squirrel Study with Smack Dab in the Middle of Maybe AIM: In Smack Dab in the Middle of Maybe, the main character Cricket discovers squirrels in a nest above the treehouse. Students can use the novel as a starting point for learning more about squirrels. OBJECTIVES: Students will be able to: 1. Identify grey squirrels. 2. Provide information about a squirrel’s nesting behavior. 3. Recognize the behavior of grey squirrels in Smack Dab in the Middle of Maybe. GET STARTED/ DO-NOW: In the novel, Cricket notices a squirrel nest above her treehouse. The nest was made by eastern grey squirrels. Have the students read the article by National Geographic about grey squirrels. Instruct them to write down five facts. https://kids.nationalgeographic.com/animals/eastern-gray- squirrel/#eastern-gray-squirrel-tree.jpg If personal computers are not available, read the article aloud. After you have finished, instruct the students to write down facts they remember. Have the students share the facts with the class. MINI-LESSON: 1. There are several species of squirrels found in North America. Eastern grey squirrels are one of them. Other species include American red squirrels and fox squirrels. Introduce these other two species by showing the class images like the ones found on these webpages: Fox Squirrel: https://www.arkive.org/eastern-fox-squirrel/sciurus-niger/ American Red Squirrel: http://www.arkive.org/american-red- squirrel/tamiasciurus-hudsonicus/ 2. Provide each student with a blank map of the United States. Together as a class, color in the areas where grey squirrels are commonly found. -

Mammal Watching in the Pacific Northwest, Summer 2019 with Notes on Birding, Locations, Sounds, and Chasing Chipmunks
Mammal watching in the Pacific Northwest, summer 2019 With notes on birding, locations, sounds, and chasing chipmunks Keywords: Sciuridae, trip report, mammals, birds, summer, July Daan Drukker 1 How to use this report For this report I’ve chosen not to do the classic chronological order, but instead, I’ve treated every mammal species I’ve seen in individual headers and added some charismatic species that I’ve missed. Further down I’ve made a list of hotspot birding areas that I’ve visited where the most interesting bird species that I’ve seen are treated. If you are visiting the Pacific Northwest, you’ll find information on where to look for mammals in this report and some additional info on taxonomy and identification. I’ve written it with a European perspective, but that shouldn’t be an issue. Birds are treated in detail for Mount Rainier and the Monterey area, including the California Condors of Big Sur. For other areas, I’ve mentioned the birds, but there must be other reports for more details. I did a non-hardcore type of birding, just looking at everything I came across and learning the North American species a bit, but not twitching everything that was remotely possible. That will be for another time. Every observation I made can be found on Observation.org, where the exact date, location and in some cases evidence photos and sound recordings are combined. These observations are revised by local admins, and if you see an alleged mistake, you can let the observer and admin know by clicking on one of the “Contact” options in the upper right panel. -

Coronado National Forest Potential Wilderness Area Evaluation Report
United States Department of Agriculture Coronado National Forest Potential Wilderness Area Evaluation Report Forest Service Southwestern Region Coronado National Forest July 2017 Potential Wilderness Area Evaluation Report In accordance with Federal civil rights law and U.S. Department of Agriculture (USDA) civil rights regulations and policies, the USDA, its Agencies, offices, and employees, and institutions participating in or administering USDA programs are prohibited from discriminating based on race, color, national origin, religion, sex, gender identity (including gender expression), sexual orientation, disability, age, marital status, family/parental status, income derived from a public assistance program, political beliefs, or reprisal or retaliation for prior civil rights activity, in any program or activity conducted or funded by USDA (not all bases apply to all programs). Remedies and complaint filing deadlines vary by program or incident. Persons with disabilities who require alternative means of communication for program information (e.g., Braille, large print, audiotape, American Sign Language, etc.) should contact the responsible Agency or USDA’s TARGET Center at (202) 720-2600 (voice and TTY) or contact USDA through the Federal Relay Service at (800) 877-8339. Additionally, program information may be made available in languages other than English. To file a program discrimination complaint, complete the USDA Program Discrimination Complaint Form, AD-3027, found online at http://www.ascr.usda.gov/complaint_filing_cust.html and at any USDA office or write a letter addressed to USDA and provide in the letter all of the information requested in the form. To request a copy of the complaint form, call (866) 632-9992. Submit your completed form or letter to USDA by: (1) mail: U.S. -

Management Indicator Species of the Kaibab National Forest: an Evaluation of Population and Habitat Trends Version 3.0 2010
Management Indicator Species of the Kaibab National Forest: an evaluation of population and habitat trends Version 3.0 2010 Isolated aspen stand. Photo by Heather McRae. Pygmy nuthatch. Photo by the Smithsonian Inst. Pumpkin Fire, Kaibab National Forest Mule deer. Photo by Bill Noble Red-naped sapsucker. Photo by the Smithsonian Inst. Northern Goshawk © Tom Munson Tree encroachment, Kaibab National Forest Prepared by: Valerie Stein Foster¹, Bill Noble², Kristin Bratland¹, and Roger Joos³ ¹Wildlife Biologist, Kaibab National Forest Supervisor’s Office ²Forest Biologist, Kaibab National Forest, Supervisor’s Office ³Wildlife Biologist, Kaibab National Forest, Williams Ranger District Table of Contents 1. MANAGEMENT INDICATOR SPECIES ................................................................ 4 INTRODUCTION .......................................................................................................... 4 Regulatory Background ...................................................................................................... 8 Management Indicator Species Population Estimates ...................................................... 10 SPECIES ACCOUNTS ................................................................................................ 18 Aquatic Macroinvertebrates ...................................................................................... 18 Cinnamon Teal .......................................................................................................... 21 Northern Goshawk ................................................................................................... -

(Tamiasciurus Hudsonicus) from Nova Scotia
Notes Records of Melanistic American Red Squirrels ( Tamiasciurus hudsonicus ) from Nova Scotia HOWARD M. H UYNH 1,2 *, B ERNARD L. B URKE 3, and DONALD F. M CALPINE 2 1 Department of Biology, Acadia University, Wolfville, Nova Scotia B4P 2R6 Canada 2 New Brunswick Museum, 277 Douglas Avenue, Saint John, New Brunswick E2K 1E5 Canada 3 21 Micmac Boulevard, Dartmouth, Nova Scotia B3A 4N3 Canada *Corresponding author: email: [email protected] Huynh, H. M., B. L. Burke, and D. F. McAlpine. 2011. Records of melanistic American Red Squirrels ( Tamiasciurus hudsonicus ) from Nova Scotia. Canadian Field-Naturalist 125(2): 15 4–157. Though melanism has been observed in several species of North American sciurids, the occurrence of this phenotype is rel - atively rare in American Red Squirrels ( Tamiasciurus hudsonicus ). We provide the first detailed accounts of melanistic Red Squirrels observed in Nova Scotia, Canada. Key Words: American Red Squirrel, Tamiasciurus hudsonicus , Melanism, Nova Scotia . Melanism in mammals refers to black or very dark of a typical adult Gray Squirrel. The presence of other brown pelage (Caro 2005), and is a relatively uncom - external characters – i.e., hair tufts protruding from the mon phenotype observed in most species of mammals top of the pinnae (more accentuated in winter pelage; (Guthrie 1967). While most populations exhibit grad - Steele 1998; Whitaker and Hamilton 1998) and small - ual intraspecific colour variation across their geo - er, flatter tail (Steele 1998) – also confirmed that this graphic range, there are some populations that show animal was a Red Squirrel. discontinuous variation (Caro 2005), with melanistic On March 22, 2010, the second author observed and (melanic) individuals occurring at high frequency photographed a young adult melanistic Red Squirrel (Guthrie 1967). -

December 22, 2010 Department Of
December 22, 2010 Department of Agriculture Secretary Tom Vilsack FAX: (202) 720-2166 Forest Service Chief Tom Tidwell FAX: (202) 205-1765 Forest Service Regional Forester Corbin Newman FAX: (505) 842-3110 Coronado National Forest Supervisor Jim Upchurch FAX: (520) 388-8305 Department of the Interior Secretary Ken Salazar FAX: (202) 208-6956 Fish and Wildlife Service Director Rowan Gould FAX: (202) 208-6965 Fish and Wildlife Service Regional Director Benjamin Tuggle FAX: (505) 248-6910 Fish and Wildlife Service State Supervisor Steve Spangle FAX: (602) 242-2513 University of Arizona President Robert Shelton FAX: (520) 621-9323 Dear Messrs. Schafer, Tidwell, Newman, Upchurch, Salazar, Gould, Tuggle, Spangle, and Shelton, RE: Notice of Intent to Sue the U.S. Department of Agriculture (USDA) and the U.S. Forest Service (USFS) for failing to reinitiate Endangered Species Act (ESA) consultation with the U.S. Fish and Wildlife Service (USFWS) regarding (1) the Mount Graham telescope project and its resulting jeopardy to the endangered Mount Graham Red Squirrel, and (2) regarding destruction of Critical Habitat. SUMMARY OF LEGAL VIOLATIONS On behalf of the Center for Biological Diversity, Maricopa Audubon Society, and the Mount Graham Coalition, I hereby provide notice, pursuant to 16 U.S.C. § 1540(g)(2), that USDA and USFS are in violation of the ESA for failing to reinitiate formal consultation under section 7 of the ESA, 16 U.S.C. § 1536, for the Mount Graham telescope project. The telescope project is permitted by USFS in consultation by USFWS. The Mount Graham telescope project has exceeded the allocated size and extent considered in its applicable July 14, 1988, USFWS Biological Opinion.