Electrowetting Using a Microfluidic Kelvin Water Dropper
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Vacuum Electrostatic Generator
1465 A VACUUM ELECTROSTATIC GENERATOR B.H. Choi, H.D. Kang, W. Kim, B.H. Oh, Korea Advanced Energy Research Institute Daeduk-Danji, Choongnam, 301-353, Korea and K.H. Chung Seoul National University Shinrim-Dong, Kwanak-Ku, Seoul, 151-741, Korea Abstract insulator supports the breakdown strength is limited to about 30 kV/cm due to the flashover phenomena on A compact electrostatic generator designed with the insulator surface. the principle of vacuum insulation has been developed. The concept of vacuum insulation instead of gas It consists of a rotating insulation disk with charge- insulation for the electrostatic generator design has carrying conductors placed around the circumference some advantages, such as the capability to hold the n n ti a non-contact induction system with R” electron high voltage in narrow inductor gap, elimination cf gun. The usable voltage of 130 kV with the generating the electrical cant acr problem by utilizing the current of about 300 pA has been obtained at the oper- non-contact induction method, posstbility of fice ational pressure of 1x10-’ torr. regulation of high voltage, and reduction cf frictional wind loss and the mechanical vibration. In Introduction addition, elimination of gas handling system may enhance the compactness and the flexibilities for the The recent progress of research and industrial accelerator system. The characteristics and the applications of ion beams require very stable beams operational performance of this vacuum electrostatic with the high energy of around 1 MeV and the current generator have been described in this paper. of a few mA. A candidate of high voltage sources to produce Experimental Apparatus mono-energetic beams is electrostatic generator, which has superior features such as small voltage ripple and The schematic drawins of the vacuum electrostatic small stored energy in the state of ultra high generator is shown in Fig. -

Basic Electrostatics System Model No
Instruction Manual Manual No. 012-07227E *012-07227* Basic Electrostatics System Model No. ES-9080 Basic Electrostatics System Model No. ES-9080 Table of Contents Equipment List........................................................... 3 Introduction .......................................................... 4-5 Equipment Description .............................................. 5-11 Electrometer...................................................................................................................................5 Electrostatics Voltage Source ........................................................................................................6 Variable Capacitor .........................................................................................................................7 Charge Producers and Proof Plane............................................................................................. 7-8 Proof Plane................................................................................................................................. 8-9 Faraday Ice Pail............................................................................................................................10 Conductive Spheres......................................................................................................................11 Resistor-Capacitor Network Accessory.......................................................................................11 Electrometer Operation and Setup Requirements................12-13 -

01. Franklin Intro 9/04
Franklin and Electrostatics- Ben Franklin as my Lab Partner A Workshop on Franklin’s Experiments in Electrostatics Developed at the Wright Center for Innovative Science Teaching Tufts University Medford MA 02155 by Robert A. Morse, Ph.D. ©2004 Sept 2004 Benjamin Franklin observing his lightning alarm. Described in Section VII. Engraving after the painting by Mason Chamberlin, R. A. Reproduced from Bigelow, 1904 Vol. VII Franklin and Electrostatics version 1.3 ©2004 Robert A. Morse Wright Center for Science Teaching, Tufts University Section I- page 1 Copyright and reproduction Copyright 2004 by Robert A. Morse, Wright Center for Science Education, Tufts University, Medford, MA. Quotes from Franklin and others are in the public domain, as are images labeled public domain. These materials may be reproduced freely for educational and individual use and extracts may be used with acknowledgement and a copy of this notice.These materials may not be reproduced for commercial use or otherwise sold without permission from the copyright holder. The materials are available on the Wright Center website at www.tufts.edu/as/wright_center/ Acknowledgements Rodney LaBrecque, then at Milton Academy, wrote a set of laboratory activities on Benjamin Franklin’s experiments, which was published as an appendix to my 1992 book, Teaching about Electrostatics, and I thank him for directing my attention to Franklin’s writing and the possibility of using his experiments in teaching. I would like to thank the Fondation H. Dudley Wright and the Wright Center for Innovative Science Teaching at Tufts University for the fellowship support and facilities that made this work possible. -

Electrostatics Lab Introductory Physics Lab Summer 2018
Washington University in St. Louis Electrostatics Lab Introductory Physics Lab Summer 2018 Electrostatics: The Shocking Truth STOP Important health warning for students with pacemakers or other electronic medical devices: This lab involves the use of a Van de Graaff generator, which produces small amounts of electrical charge. They are regularly used in elementary schools, high schools, colleges, and science centers. They pose no risk to health or safety, except to students with pacemakers or other electronic medical devices. If you use a pacemaker or other electronic medical device, please contact the Lab Manager, Merita Haxhia at [email protected], IMMEDIATELY to make other arrangements for lab. It is very unlikely that you will be affected, but safety is our top priority. As long as you do not have this type of medical device, you have absolutely nothing to worry about. However, it is not recommended that you bring sensitive electronic equipment to this experiment. Pre-Lab: The Van de Graaff Generator A Bit of History The Van de Graaff generator is an electrostatic generator, capable of producing constant electric potential differences reaching about 10 million volts. The model that you will use (thankfully) only achieves about 1% of that. The term Van de Graaff electrostatic generator may sound a little foreign, but there is one electrostatic generator that you are no doubt familiar with: earth’s atmosphere. In fact, some of the most famous experiments in the history of electrostatics were done using the atmosphere. In May of 1752, Benjamin Franklin performed his well-known kite experiment, an experiment which strongly suggested that lightning might not be so different from the sparks he produced using silk and glass. -

Experiment Using Electrostatic Motor
Experiment Using Electrostatic Motor 1. Learning Outcome In this sub-unit, we will perform experiment related to the attractive and repulsive forces of static electricity (Coulomb force) using the Electrostatic Motor (Franklin Motor) and Static Genecon. As the typical principle of static electricity, we will confirm the phenomena of electrostatic induction, attractive and repulsive forces. Let’s start our experiment for the sake of analyzing this phenomenon. 2. Historical Background In this sub-unit, we use an Electrostatic Motor, namely Franklin Motor, which was first invented by Benjamin Franklin and Andrew Gordon between 1740s and 1750s. Electrostatic Motor is based on the principles of electrostatic induction, attractive and repulsive force. Electrostatic Motor feature is operation with high voltage and low current. On the contrary, other types of (normal) motors can be operated with low voltage and large current, because their principle is electromagnetic induction. 3. Electrostatic Generator: “Static Genecon” We already know that if we rub piece of plastic with felt or different kind of cloth then static electricity will be generated. And we have in various ways confirmed properties of above mentioned way of generating static electricity. As a result, we have learned among other things as well, that electrostatic charge has two kinds. Furthermore, we can store static electricity because of the invention of Leyden jar and Electrophorus. By using them we can store greater 1 © Narika Corporation 2020 amount of static electricity, thus conducting experiments with large amount of static electricity. Because of that invention research about static electricity accelerated in the past. In 1929, Robert J. -

Basic Electrostatics System Model No
Instruction Manual Manual No. 012-07227D Basic Electrostatics System Model No. ES-9080A Basic Electrostatics System Model No. ES-9080A Table of Contents Equipment List........................................................... 3 Introduction .......................................................... 4-5 Equipment Description .............................................. 5-11 Electrometer...................................................................................................................................5 Electrostatics Voltage Source ........................................................................................................6 Variable Capacitor .........................................................................................................................7 Charge Producers and Proof Plane............................................................................................. 7-8 Proof Plane................................................................................................................................. 8-9 Faraday Ice Pail............................................................................................................................10 Conductive Spheres......................................................................................................................11 Resistor-Capacitor Network Accessory.......................................................................................11 Electrometer Operation and Setup Requirements................12-13 Suggested -

Theory of Triboelectric Nanogenerators for Self-Powered Systems
THEORY OF TRIBOELECTRIC NANOGENERATORS FOR SELF- POWERED SYSTEMS A Dissertation Presented to The Academic Faculty by Simiao Niu In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the School of Materials Science and Engineering Georgia Institute of Technology May 2016 COPYRIGHT © SIMIAO NIU 2016 THEORY OF TRIBOELECTRIC NANOGENERATORS FOR SELF- POWERED SYSTEMS Approved by: Dr. Zhong Lin (Z.L.) Wang, Advisor Dr. Russell D. Dupuis School of Materials Science and School of Electrical and Computer Engineering Engineering Georgia Institute of Technology Georgia Institute of Technology Dr. Meilin Liu Dr. Farrokh Ayazi School of Materials Science and School of Electrical and Computer Engineering Engineering Georgia Institute of Technology Georgia Institute of Technology Dr. John D. Cressler School of Electrical and Computer Engineering Georgia Institute of Technology Date Approved: February 4th, 2016 To my beloved families and friends ACKNOWLEDGEMENTS First, I would like to express my sincere thanks to my Ph.D. advisor, Dr. Zhong Lin Wang, for his visional research guidance and wonderful care on my life. I still remember when I first came to the Georgia Institute of Technology, I was only 20 years old. From then on, Dr. Wang gave me his endless love, which helped me grow up both in my research and in my personal characteristics. I also want to express my sincere thanks to my committee members, including Dr. Meilin Liu, Dr. John D. Cressler, Dr. Farrokh Ayazi and Dr. Russell D. Dupuis, for their insightful advice on my doctoral research and thesis, without which the quality of this dissertation cannot be guaranteed. -

Accelerators Overview
Introduction to Accelerators Overview Saturday Morning Physics Fermilab – October 18th Particle accelerators Particle accelerator is a device that uses electrostatic force to accelerate and electromagnetic fields to bend charge particles to high speeds and energy SMP - Introduction to Accelerators | fgg 2 What is a particle accelerator? Does anyone knows what . World’s biggest particle accelerator the name of this machine is? . 7 TeV energy , 17 miles (27 km) in circumference . 574 ft (~180 m) buried underground between the border of France and Switzerland heat matter at temperatures last seen since Big Bang LHC: Ulra High vacuum Order the magnitude lower than ISS space station Very low temperatures -456o F SMP - Introduction to Accelerators | fgg 3 How many accelerators are there? There are > 15,000 particle accelerators around the world Only research particle accelerators are shown here. Data: ELSA [http://www-elsa.physik.uni-bonn.de/accelerator_list.html] SMP - Introduction to Accelerators | fgg 4 How do they work? Which of these particles you cannot put into an accelerator? Required elements to make an accelerator Particles Energy Control Collision Detector e n p g l e r o e u o l c t t d t r o r o n a o n t n o m SMP - Introduction to Accelerators | fgg 5 Particle accelerators Modern Physics Computing Quantum Physics Accelerator design code Particle Physics High performance computing Engineering Radio Frequency Classical Physics Alignment & Survey Ultra high vacuum Mechanics Particle Electronics Electrodynamics Accelerator Magnet Technology Mechanical Cryogenics SMP - Introduction to Accelerators | fgg 6 A little bit about me… This is Originally from Sao Paulo (USP), Brazil great… What do I like… BRR!!! soccer, football, F1, ski, hiking, camping, Fermilab… relax on the beach…21F Here I come! Interval training workout Movies, concerts, plays, operas ~8,400Education miles – University later of Sao Paulo 1995 Ms in Theoretical Particle Physics 2000 PhD in Exp. -

Low Cost Electrostatic Generators Made from Funflystick™ Toy
Apparatus Competition 2009 AAPT Summer Meeting Ann Arbor, MI Low cost Electrostatic generators made from FunFlyStick™ toy Robert A. Morse St. Albans School, Mount St. Albans, Washington, DC 20016 202-537-6452 [email protected] Abstract The Fun Fly Stick™ electrostatics toy (about $30 from various sources) can be easily modified for use as an inexpensive table-top Van de Graaff generator, or as a dipole electrostatics generator for experiments with electrostatic circuits. The modified generator reaches about 60 kV, has a capacitance of about 6pF and is equivalent to a VDG with a diameter of about 12 cm. Construction of Apparatus: The Fun Fly Stick™ is a hand held Van de Graaff generator (VDG) run by two AA cells, with an internal belt giving a positive charge to a cardboard collector. Its intended use is to levitate lightweight shapes of aluminized Mylar™. The generator ground is a ring around the push button switch on the handle which grounds it to the operator. The generator charges the cardboard collector positively relative to ground. A small DC power supply (3V, 2 A) could be used to replace the batteries if desired. To convert it to a table-top VDG, remove the cardboard collector tube and replace it with an empty 23.5 oz pop top aluminum drink can (e.g. Arizona™iced tea can) pushing the mouth of the can over the end of the Fun Fly Stick™ with the can tab contacting the collector brush on the Fun Fly Stick™. Wrap the handle with heavy duty aluminum foil so the foil covers the push button switch. -
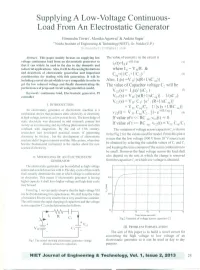
Supplying a Low-Voltage Continuous- Load from an Electrostatic Generator
Supplying A Low-Voltage Continuous- Load From An Electrostatic Generator Himanshu Tiwari', Monika Agarwal' & Ankita Sagar' '.3Noida Institute of Engineering & Technology(NIET), Gr. Noida (V.P.) [email protected] Abstract- This paper mainly focuses on supplying low The value of current i, in the circuit is voltage continuous load from an electrostatic generator so i (t)=lo e-tl(R.Ceq) (I) that it can widely be used in the day to day domestic and l industrial applications. Also, it will be discussing limitations where 10 = V HIR. & and drawback of electrostatic generation and important c,=(1/C r+ 1/C 2yl (2) consideration for dealing with this generation. It will be including a novel circuit which is very compatible in order to Also, lICs) =V H/{s(R+lIsCeq)} get the low reduced voltage and finally demonstrating the The value of Capacitor voltage C2 will be performance of proposed circuit using simulation model. VC2(s) = l\(s)1 (sC2) (3) Keywords: continuous load, Electrostatic generator, PI controller VC2(s) = V HI {s(R+ 1/sC eq)}· 1/(sC 2) VC2(s) = V HI Ci {S2 . (R+ lIsC eq)} I. INTRODUCTION = V H' Ce/C2· lI{S (S +1IRC eq)} An electrostatic generator or electrostatic machine is a v clt) = V H' C glC • [1- e-tl(R.Ceq)] (4) mechanical device that produces static electricity or electricity e 2 at high voltage, however, at low power levels. The knowledge of Ifvalue of t « RC eq: VC2(t) = 0 static electricity was discussed in mid sixteenth century but eq: merely as an interesting and mystifying phenomenon and often Ifvalue oft» RC VC2(t)=VH. -

Karen Aplin1, Giles Harrison2, Jeff Lidgard1 and Graeme Marlton2
Demonstrating atmospheric electrification phenomena Karen Aplin1, Giles Harrison2, Jeff Lidgard1 and Graeme Marlton2 1 Physics Department, University of Oxford, Keble Road, Oxford OX1 3RH UK 2 Department of Meteorology, University of Reading, Earley Gate, Reading RG6 6BB UK 1. Abstract 5. Lightning location Lightning is both widely experienced and commonly recognised as During the late nineteenth and early twentieth centuries, it was electrical in origin. Studying atmospheric electrical effects in the realised that the large and fluctuating electrical currents associated classroom, however, generally requires high voltage sources and in with lightning were responsible for brief but broad-band radio signals some cases may not be possible. Generating high voltages through known as sferics (a contraction of the word ‘atmospherics’). These can electrostatics can provide good and controllable laboratory be heard as brief clicks and pops on an AM radio during a storm. demonstrations, through which phenomena related to lightning Scientists in the UK exploited this characteristic to locate lightning. As discharges can be experienced and explained. This adds an their knowledge grew, their apparatus became more sophisticated, experiential element to education in atmospheric electricity that can Fig 2 (left) A Wimshurst machine. Disks with multiple coupon electrodes counter- eventually forming the modern ATD (Arrival Time Difference) lightning be explained at a variety of technical levels, from school children to rotate,. and generate a substantial charge by friction (middle) View of the electrodes, detection system now run by the UK Met Office. The basic principles the general public to final year undergraduate students. the spacing between which can be varied to find the breakdown distance at are simple however. -

Electrostatic Application Principles
International PhD Seminar on Computational electromagnetics and optimization in electrical engineering – CEMOEE 2010 10-13 September, Sofia, Bulgaria ELECTROSTATIC APPLICATION PRINCIPLES invited paper Mićo GAĆANOVIĆ "Jede Ursache hat ihre Wirkung; jede Wirkung ihre Ursache; an explanation of ultimate substance, change, and the exis- alles geschieht gesetzmäßig, Zufall ist nur ein Name für ein tence of the world -- without reference to mythology. Those unbekanntes Gesetz. Es gibt viele Ebenen der Ursächlichkeit, aber philosophers were also influential, and eventually Thales' nichts entgeht dem Gesetz." rejection of mythological explanations became an essential “Kybalion” idea for the scientific revolution. He was also the first to define general principles and set forth hypotheses, and as a result has been dubbed the "Father of Science". HISTORY In mathematics, Thales used geometry to solve prob- lems such as calculating the height of pyramids and the Thales of Miletus Thales of Miletus (Θαλῆς ὁ distance of ships from the shore. He is credited with the Μιλήσιος) Born ca. 624–625 BC Died ca. 547–546 BC , first use of deductive reasoning applied to geometry, by [16]. deriving four corollaries to Thales' Theorem. As a result, he As reported by the Ancient Greek philosopher Thales of has been hailed as the first true mathematician and is the Miletus around 600 B.C.E., charge (or electricity) could be first known individual to whom a mathematical discovery accumulated by rubbing fur on various substances, such as has been attributed. amber. The Greeks noted that the charged amber buttons In 1600, the English scientist William Gilbert returned could attract light objects such as hair.