151650-Medadnews-Aug
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
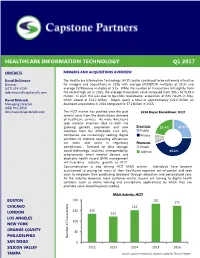
Capstone Healthcare IT M&A Report Q1 2017
HEALTHCARE INFORMATION TECHNOLOGY Q1 2017 CONTACTS MERGERS AND ACQUISITIONS OVERVIEW David DeSimone The Healthcare Information Technology (HCIT) sector continued to be extremely attractive Director for mergers and acquisitions in 2016 with average EV/EBITDA multiples of 23.9x and (617) 619‐3324 average EV/Revenue multiples of 5.3x. While the number of transactions fell slightly from [email protected] the record high set in 2015, the average transaction value increased from $95.5 to $149.4 million. In part, this was due to Quintiles’ blockbuster acquisition of IMS Health in May, David Michaels which closed at $13.2 billion. Buyers spent a total of approximately $25.8 billion on Managing Director disclosed acquisitions in 2016 compared to $7.2 billion in 2015. (858) 926‐5950 [email protected] The HCIT market has profited over the past 2016 Buyer Breakdown: HCIT several years from the downstream demand of healthcare services. As more Americans seek medical attention (due to both the growing geriatric population and new STRATEGIC 21.4% 24.9% enrollees from the Affordable Care Act), Public companies are increasingly seeking digital Private solutions to improve operating efficiencies, 8.7% cut costs and assist in regulatory FINANCIAL compliances. Demand for data storage, Direct cloud technology, analytics, interoperability, Add‐on 45.1% cybersecurity, smart medical devices and electronic health record (EHR) management will help drive industry growth in 2017. Consumerization is also driving HCIT M&A activity. Individuals have become accustomed to paying for many of their healthcare expenses out‐of‐pocket and seek ways to empower their purchasing decisions through education and personalized care. -

Rank1/200) (Rank: 6/200) Rank Company Number Avg
493 Total International Students On Campus Spring 2103 272 Total International COB Students Spring 2013 55% of Total International Student Population 18% of COB Population Computer Systems Analyst Management Analysts (Rank1/200) (Rank: 6/200) Rank Company Number Avg. Salary Rank 1 Infosys Limited 10,154 $73,412 1 2 Cognizant Technology Solutions 1,761 $67,051 2 3 IBM 1,217 $85,232 3 4 Deloitte Consulting 1,111 $79,778 4 5 Satyam Computer Services 1,076 $72,356 5 6 UST Global 1,013 $63,886 6 7 HCL Technologies America 977 $73,009 7 8 Patni Americas 820 $70,699 8 9 Wipro 785 $82,443 9 10 Hexaware Technologies 610 $63,204 10 11 Deloitte Touche 549 $81,179 11 12 Accenture 491 $79,527 12 13 Tata Consultancy Services 381 $65,010 13 14 Mphasis 367 $66,044 14 15 Synechron 358 $77,189 15 16 Capgemini Financial Services 318 $78,030 16 17 Virgo 291 $60,227 17 18 Hewlett Packard 272 $96,771 18 19 Diaspark 270 $64,728 19 20 Yash Technologies 269 $55,205 20 21 Advent Global Solutions 247 $62,220 21 22 RS Software India 243 $67,253 22 23 Infosys Limited 241 $78,296 23 24 Persistent Systems 240 $71,556 24 25 Reliable Software Resources 232 $60,521 25 26 Larsen Toubro Infotech 231 $65,177 26 27 kforce 225 $90,304 27 28 Ernst Young 217 $89,948 28 29 Pricewaterhousecoopers 208 $66,478 29 30 Compunnel Software Group 206 $76,062 30 31 Polaris Software Lab 204 $69,457 31 32 Tech Mahindra americas 204 $64,965 32 33 Horizon Technologies 199 $61,330 33 34 SAP America 198 $102,811 34 35 Orian Engineersorporated 197 $62,835 35 36 Oracle 195 $93,968 36 37 Syntel -

2008 Umaine News Press Releases
The University of Maine DigitalCommons@UMaine General University of Maine Publications University of Maine Publications 2008 2008 UMaine News Press Releases Division of Marketing and Communications Joe Carr University of Maine George Manlove University of Maine Dan Cashman University of Maine Margaret Nagle University of Maine Follow this and additional works at: https://digitalcommons.library.umaine.edu/univ_publications Part of the Higher Education Commons, and the History Commons Repository Citation Division of Marketing and Communications; Carr, Joe; Manlove, George; Cashman, Dan; and Nagle, Margaret, "2008 UMaine News Press Releases" (2008). General University of Maine Publications. 1092. https://digitalcommons.library.umaine.edu/univ_publications/1092 This Monograph is brought to you for free and open access by DigitalCommons@UMaine. It has been accepted for inclusion in General University of Maine Publications by an authorized administrator of DigitalCommons@UMaine. For more information, please contact [email protected]. UMaine News Press Releases from Word Press XML export 2008 Williams Appointed to University of Maine Foundation Post 07 Jan 2008 Contact: Contact: Amos Orcutt, University of Maine Foundation President/CEO, 207 581-5100 ORONO -- Amos Orcutt, president/CEO of the University of Maine Foundation, has announced the appointment of Daniel B. Williams as planned giving officer. Williams, who earned both a bachelor's degree and a master's degree from UMaine, returns to his alma mater having accumulated a great deal of experience relevant to his new position. Since 2006, Williams has served as foundation president and director of development for Eastern Maine Community College in Bangor. In that role, he was responsible for all institutional fundraising including planned giving, annual and endowed scholarships, in-kind gifts, annual campaign and capital projects. -

J2 Global Annual Report 2021
J2 Global Annual Report 2021 Form 10-K (NASDAQ:JCOM) Published: March 1st, 2021 PDF generated by stocklight.com UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K ☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2020 OR ☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission File Number: 0-25965 J2 GLOBAL, INC. (Exact name of registrant as specified in its charter) Delaware 47-1053457 (State or other jurisdiction of incorporation or organization) (I.R.S. Employer Identification No.) 700 S. Flower Street, 15th Floor, Los Angeles, California 90017, (323) 860-9200 (Address and telephone number of principal executive offices) Securities registered pursuant to Section 12(b) of the Act: Title of each class Trading Symbol(s) Name of each exchange on which registered Common Stock, $0.01 par value JCOM Nasdaq Stock Market LLC Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐ Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒ Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -

Core Investor Presentation August 11, 2020 Safe Harbor for Forward-Looking Statements
Core Investor Presentation August 11, 2020 Safe Harbor for Forward-Looking Statements Certain statements in this presentation are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, particularly those regarding our 2020 Financial Guidance. Such forward-looking statements are subject to numerous assumptions, risks and uncertainties that could cause actual results to differ materially from those described in those statements. Readers should carefully review the Risk Factors slide of this presentation. These forward-looking statements are based on management’s expectations or beliefs as of August 11, 2020 as well as those set forth in our Annual Report on Form 10-K filed by us on March 2, 2020 with the Securities and Exchange Commission (“SEC”) and the other reports we file from time to time with the SEC. We undertake no obligation to revise or publicly release any updates to such statements based on future information or actual results. Such forward-looking statements address the following subjects, among others: • Future operating results • Ability to acquire businesses on acceptable terms and integrate and recognize synergies from acquired businesses • Deployment of cash and investment balances to grow the company • Subscriber growth, retention, usage levels and average revenue per account • Cloud service and digital media growth and continued demand for fax services • International growth • New products, services, features and technologies • Corporate spending including stock repurchases • Intellectual property and related licensing revenues • Liquidity and ability to repay or refinance indebtedness • Systems capacity, coverage, reliability and security • Regulatory developments and taxes All information in this presentation speaks as of August 11, 2020 and any redistribution or rebroadcast of this presentation after that date is not intended and will not be construed as updating or confirming such information. -

J2 GLOBAL, INC. (Exact Name of Registrant As Specified in Its Charter) Delaware 47-1053457 (State Or Other Jurisdiction of Incorporation Or Organization) (I.R.S
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2016 OR TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission File Number: 0-25965 j2 GLOBAL, INC. (Exact name of registrant as specified in its charter) Delaware 47-1053457 (State or other jurisdiction of incorporation or organization) (I.R.S. Employer Identification No.) 6922 Hollywood Boulevard, Suite 500, Los Angeles, California 90028, (323) 860-9200 (Address and telephone number of principal executive offices) Securities registered pursuant to Section 12(b) of the Act: None Securities registered pursuant to Section 12(g) of the Act: Common Stock, $0.01 par value (Title of class) Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes No Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes No Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes No Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). -
The IT Manager's Survival Guide
White Paper The IT Manager’s Survival Guide: Outsource Your Fax Infrastructure to the Cloud White Paper The IT Manager’s Survival Guide: Outsource Your Fax Infrastructure to the Cloud Even if you’ve moved to a more This how-to guide will give you an overview (advantages and drawbacks) of today’s prevailing sophisticated on premises fax enterprise fax technologies, to help you determine — based on your needs, existing cost structure and IT infrastructure, with internally overhead — whether a cloud fax solution is right for managed fax servers, are you you, versus continuing to maintain and support on- site or ‘hybrid’ fax infrastructure. After your analysis, realizing maximum efficiency and if you decide that cloud faxing is the way to go, this guide will help you select the right cloud fax service cost-effectiveness from this solution? provider. A CRITICAL LOOK AT THE THREE Relative to the many demands on today’s IT teams, TYPES OF ENTERPRISE FAX spending capital, time and resources to maintain physical fax servers and infrastructure is not a SOLUTIONS high priority. But fax capability remains a business It may surprise you to learn that millions of need simply because many established industries businesses rely on faxing to transmit their most — such as legal, healthcare, financial services and important documents — even many in the high- manufacturing — are dependent on the security, tech industry. And many are facing the same critical reliability and process integration of their fax questions you may be considering today: infrastructure. With this in mind, evaluating new fax technologies, which can help to balance business • Even if you’ve moved to a more sophisticated needs against the demands on IT — and to meet on-premises fax infrastructure, with internally the service-level requirements for internal and managed fax servers, are you realizing maximum external consumers of electronically transmitted fax efficiency and cost-effectiveness from this documents — is a wise investment of your time. -

Reach Consensus with J2 Global's New Interoperability Platform
Reach Consensus with J2 Global’s New Interoperability Platform Leading Cloud Fax Company Offers a Comprehensive, Connected Solution for Better Continuum of Care Los Angeles, CA – February 28, 2020 -- J2 Global, Inc. (NASDAQ: JCOM) the leader in digital cloud faxing, announced today an unparalleled opportunity for healthcare providers to move from paper faxing to a comprehensive interoperability system through an easy-to-use platform that connects healthcare organizations through the entire continuum-of-care. Called Consensus, the platform offers one comprehensive connection with a simple, inbox-like dashboard to manage all incoming and outgoing patient documents including cloud faxes, direct messaging, patient query and API integration into exchanges. Users do not need an EHR to provide this level of enhanced data fluidity and digital interoperability. Those with an EHR will find an easy workflow for integrating patient data. Consensus provides healthcare organizations access to patient data from a single dashboard for: ● Digital cloud faxing — as easy as sending and receiving email — without the need for paper, fax lines, or on-prem fax hardware. ● Conversion of a cloud fax phone number to send documents as a direct message. ● Direct messaging over a secure network with access to over a million provider addresses. ● Patient queries via CommonWell and Carequality. ● Connectivity access to referral networks, ACOs, community and state exchanges, and HIEs. ● Support of standards such as C-CDA, HL7, and FHIR. "Having a user-friendly workspace with the ability to manage patient health records for cloud faxing, direct message and record based query would help our organization expand standardly into varied health environments with confidence to support the workflow and uneven user needs and technical capabilities," said Thalia Baker, Associate Vice President, Primary Care at UAB Medicine. -

J2 Global 2020 Annual Diversity Report
J2 Global 2020 Annual Diversity Report 3 Introduction 6 Where We Are Now 7 Workforce Representation 12 Hiring & Inclusivity Contents 14 Senior Leadership 17 Corporate Leadership & Board 18 Education & Philanthropy 20 What’s Next 22 Appendix © 2020 J2 Global, All Rights Reserved Diversity at J2 Global | 2 Differences make us stronger. They enable us to have a more expansive view and understanding of the communities we serve. They allow for breakthroughs and innovation. They empower us to be adaptable to change. J2 Global unequivocally embraces the business and societal imperative to have a diverse and inclusive organization. It has been a fundamental priority, and this Diversity Report demonstrates some of the progress we’ve made and the important work that still needs to be done. We need to be “We need to be impatient, responsible for our own workforce and leadership diversity force action and be and leverage our power and platforms to educate and influence our audiences and partners around the world. unwilling to compromise in our pursuit of diversity, We need to be impatient, force action and be unwilling to compromise in our pursuit of diversity, equity and inclusion. equity and inclusion.” Thank you for playing an important role in this journey. Vivek Shah Chief Executive Officer © 2020 J2 Global, All Rights Reserved Diversity at J2 Global | 3 Diversity, equity and inclusion matter to us at J2 Global, and we believe that “Doing is Greater Than Talking.” We are taking actions today to build and sustain a global employee population that mirrors the diverse communities we serve around the world. -

THE INFORMATION NEEDS of COMMUNITIES the Changing Media Landscape in a Broadband Age
THE INFORMATION NEEDS OF COMMUNITIES The changing media landscape in a broadband age Steven Waldman and the Working Group on Information Needs of Communities Federal Communications Commission JULY 2011 www.fcc.gov/infoneedsreport a b THE INFORMATION NEEDS OF COMMUNITIES The changing media landscape in a broadband age Steven Waldman and the Working Group on Information Needs of Communities Federal Communications Commission Table of Contents Executive Summary and Overview 5 A Large Number of Stations Do No News at All Network news PART ONE THE MEDIA LANDscAPE Cable Cable News Networks Section 1. Commercial Media 33 Local Cable News 1. NEwsPAPERS 34 Cable Trends Early History: Cheap Paper, the Telegraph, and the Rise of Satellite the Independent Press Current State The First Technological Challenges: Radio and TV The Rise of the Lucrative Monopoly Newspaper 4. INTERNET 116 The Next Technological Challenge: The Internet How the Internet Has Improved Journalism Was the Decline of Newspapers Inevitable? More Diversity and Choice Hamsterization Greater Depth The Price of Newspaper Cuts More Diversity in Commentary and Analysis Going Forward Enabling Citizen Engagement Speed and Ease 2. RADIO 58 Expanding Hyperlocal Coverage The Birth of Radio News Serving Highly Specific Interests Deregulation Cheaper Content Distribution The Current State of Radio Cheaper Content Creation Local News Radio Direct Access to Community and Civic News The Rise of News/Talk However, the Internet Has Not Solved Some of The Changing Radio Market Journalism’s Key Problems -

2020 J2 Global Analyst Day Presentation
Analyst Day Presentation March 4, 2020 1 Safe harbor for forward-looking statements Certain statements in this presentation are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, particularly those regarding our 2020 Financial Guidance. Such forward-looking statements are subJect to numerous assumptions, risks and uncertainties that could cause actual results to differ materially from those described in those statements. Readers should carefully review the Risk Factors slide of this presentation. These forward-looking statements are based on management’s expectations or beliefs as of March 4, 2020 as well as those set forth in our Annual Report on Form 10-K filed by us on March 2, 2020 with the Securities and Exchange Commission (“SEC”) and the other reports we file from time to time with the SEC. We undertake no obligation to revise or publicly release any updates to such statements based on future information or actual results. Such forward-looking statements address the following subJects, among others: • Future operating results • Ability to acquire businesses on acceptable terms and integrate and recognize synergies from acquired businesses • Deployment of cash and investment balances to grow the company • Subscriber growth, retention, usage levels and average revenue per account • Cloud service and digital media growth and continued demand for fax services • International growth • New products, services, features and technologies • Corporate spending including stock repurchases • Intellectual property and related licensing revenues • Liquidity and ability to repay or refinance indebtedness • Systems capacity, coverage, reliability and security • Regulatory developments and taxes All information in this presentation speaks as of March 4, 2020 and any redistribution or rebroadcast of this presentation after that date is not intended and will not be construed as updating or confirming such information. -

J2 Global 2021 Annual Diversity Report
J2 Global 2021 Annual Diversity Report 3 Introduction 6 Where We Are Today 7 Workforce Representation 13 Hiring & Inclusivity 17 Senior Leadership 20 Promotions Overview Contents 21 Corporate Leadership 22 Board of Directors 23 Our Pandemic Response 25 Education & Philanthropy 30 Building Community Through ERGs 34 Listening To Our Employees; Amplifying Their Voices 35 What’s Next—Our Commitments © 2021 J2 Global, All Rights Reserved Diversity at J2 Global | 2 Last year, we embraced the mantra that “Doing is Greater than Talking.” We shared this commitment widely, reminding our employees of it as we continued our outreach in our communities, made changes, launched programs, and empowered leaders from within to build a better, stronger, more diverse J2 Global. Our employees took that commitment to heart, getting involved at all levels of our organization — speaking up, stepping up, taking action. We applaud their efforts and are “Our employees took that proud of the progress we have made in encouraging a diverse commitment to heart, and inclusive workplace, even as we all shifted together to a virtual one. This Diversity Report highlights that progress, as getting involved at all well as where we’re coming up short. levels of our organization Without question, there is still a great deal of work to do and — speaking up, stepping we are all accountable for continued improvement. We must up, taking action.” continue in our proactive efforts to diversify our workforce; we must continue to ensure an equitable and inclusive J2; we must continue to act with urgency. Thank you for joining us in this essential effort.