Yeast Extract Including Disodium Inosinate Salt and Disodium Guanylate Salt and Producing Method Thereof
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stress Condition on a Restricted Sodium Diet Using Umami Substance (L-Glutamate) in a Pilot Randomized Cross-Over Study
foods Article Stress Condition on a Restricted Sodium Diet Using Umami Substance (L-Glutamate) in a Pilot Randomized Cross-Over Study Tamami Iwamoto 1,* , Andrea Wakita 2, Saiko Shikanai 3, Hideki Matsumoto 2, Mariko Hirota 2, Hisayuki Uneyama 2, Vu Thi Thu Hien 4 and Shigeru Yamamoto 1 1 Department of Food and Nutritional, Faculty of Human Life, Jumonji University, Saitama 352-8510, Japan; [email protected] 2 Ajinomoto Co., Inc., Kanagawa 210-8681, Japan; [email protected] (A.W.); [email protected] (H.M.); [email protected] (M.H.); [email protected] (H.U.) 3 Asian Nutrition and Food Culture Research Center, Saitama 352-8510, Japan; [email protected] 4 National Institute of Nutrition, Hanoi 84, Vietnam; [email protected] * Correspondence: [email protected]; Tel.: +81-48-477-0555; Fax: +81-48-478-9367 Abstract: Hypertensive patients who adopt a sodium-restricted diet have difficulty maintaining this change, and this could increase stress. On the other hand, soup rich in umami substances (dashi) was reported to reduce indexes of anxiety and stress. The objective of this study was to measure mood and physiological stress indexes during administration of a sodium-restricted diet with and without an umami substance (free L-glutamate) by a cross-over randomized, single-blind, placebo-controlled trial in Japanese female university students. The baseline was measured for 5 days followed by Citation: Iwamoto, T.; Wakita, A.; a sodium-restricted diet intervention phase that lasted for 10 days. The Profile of Mood States Shikanai, S.; Matsumoto, H.; Hirota, questionnaire was administered, a stress marker in saliva (chromogranin-A) was measured, and the M.; Uneyama, H.; Hien, V.T.T.; amount of sodium intake was confirmed from 24 h urine collection samples. -

Low Acyl Gellan Gum for Inclusion on the National List of Substances Allowed in Organic Production and Handling (7 CFR 205.605 (B)
Petition for Evaluation of Low Acyl Gellan Gum for Inclusion on the National List of Substances Allowed in Organic Production and Handling (7 CFR 205.605 (b) Submitted by: CP Kelco U.S., Inc. 3100 Cumberland Blvd., Suite 600 Atlanta, GA 30339 Date: 08 August 2019 CP Kelco U.S., Inc. 08 August 2019 National Organic List Petiion Low Acyl Gellan Gum Table of Contents Item A.1 — Section of National List ........................................................................................................... 4 Item A.2 — OFPA Category - Crop and Livestock Materials .................................................................... 4 Item A.3 — Inert Ingredients ....................................................................................................................... 4 1. Substance Name ................................................................................................................................... 5 2. Petitioner and Manufacturer Information ............................................................................................. 5 2.1. Corporate Headquarters ................................................................................................................5 2.2. Manufacturing/Processing Facility ...............................................................................................5 2.3. Contact for USDA Correspondence .............................................................................................5 3. Intended or Current Use .......................................................................................................................5 -

(12) Patent Application Publication (10) Pub. No.: US 2003/0198723 A1 Kuroda Et Al
US 2003O198723A1. (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0198723 A1 Kuroda et al. (43) Pub. Date: Oct. 23, 2003 (54) SEASONING COMPOSITIONS, FOODS AND Related U.S. Application Data DRINKS WITH THE USE THEREOF AND PROCESSES FOR PRODUCING THE SAME (63) Continuation of application No. PCT/JP01/03174, filed on Apr. 12, 2001. (75) Inventors: Motonao Kuroda, Kawasaki-shi (JP); Fumihiko Odashima, Tokyo (JP); (30) Foreign Application Priority Data Toshihito Seki, Kawasaki-shi (JP) Apr. 24, 2000 (JP)...................................... 2OOO-1221.69 Correspondence Address: Sep. 29, 2000 (JP)...................................... 2000-297859 OBLON SPIVAK MCCLELLAND MAIER & Mar. 29, 2001 (JP)...................................... 2001-095859 NEUSTADT Publication Classification FOURTH FLOOR 1755JEFFERSON DAVIS HIGHWAY (51) Int. Cl." ....................................................... A23C 1/00 ARLINGTON, VA 2.2202 (US) (52) U.S. Cl. .............................................................. 426/442 (73) Assignee: AJINOMOTO CO., INC., Tokyo (JP) (57) ABSTRACT (21) Appl. No.: 10/277,806 The present invention relates to a Seasoning composition that Suppresses unpleasant acidic tastes or acidic Smells present in or generated by foods and drinks, especially (22) Filed: Oct. 23, 2002 foods, and a process for producing the Same. US 2003/O198723 A1 Oct. 23, 2003 SEASONING COMPOSITIONS, FOODS AND ciated with acetic acid or a similar acid, present in or DRINKS WITH THE USE THEREOF AND generated by foods and drinks, especially pre-cooked daily PROCESSES FOR PRODUCING THE SAME foods. 0009. Another object of the present invention is to pro CROSS REFERENCE TO RELATED vide foods and drinks, in particular pre-cooked daily-foods, APPLICATIONS that have excellent preservation properties and have a favor 0001. -

(12) Patent Application Publication (10) Pub. No.: US 2005/0025877 A1 Cuenca Et Al
US 2005.0025877A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0025877 A1 Cuenca et al. (43) Pub. Date: Feb. 3, 2005 (54) HYDROLYZED VEGETABLE PROTEIN (22) Filed: Jul. 8, 2004 LIQUID COMPOSITIONS (30) Foreign Application Priority Data (75) Inventors: Lynn M. Cuenca, Caloocan City (PH); Christina Rowena S. Bautista, Jul. 28, 2003 (PH) - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 120O3OOO324 Batangas (PH); Flora H. Alvarez, Bulacan (PH); Kennie U. Dee, Quezon Publication Classification City (PH) (51) Int. Cl." ....................................................... A23L 1/10 Correspondence Address: (52) U.S. Cl. .............................................................. 426/650 BROWN & MICHAELS, PC 400 M & T BANK BUILDING 118 NORTH TOGA ST (57) ABSTRACT ITHACA, NY 14850 (US) Good tasting hydrolyzed vegetable protein liquid Seasoning (73) Assignee: NUTRI-ASIA, INC., Mandaluyong City compositions where the vegetable protein has been hydro (PH) lyzed with sulfuric acid. The novel compositions address the bitter taste associated with Sulfuric acid-hydrolyzed veg (21) Appl. No.: 10/887,487 etable proteins. US 2005/0025877 A1 Feb. 3, 2005 HYDROLYZED VEGETABLE PROTEIN LIQUID 0010. The vegetable protein sources include, among oth COMPOSITIONS ers, corn, rice, wheat, Soy, nuts, and mixtures thereof. The preferred vegetable protein Source is Soybean. REFERENCE TO RELATED APPLICATIONS 0001. This application claims an invention which was 0011. The flavor enhancer is a mixture consisting of disclosed in Republic of the Philippines Patent Application disodium 5'-inosinate and disodium 5'-guanylate, a common No. 12003000324, filed Jul. 8, 2003, entitled “HYDRO flavor enhancer in the industry. The mixture could also come LYZED VEGETABLE PROTEIN LIOUID COMPOSI from natural Sources, Such as, for example, yeast extract. -

Glutamate and Excitotoxin Food List
Glutamate and Excitotoxin Food List Much of this information comes from the work of Russell Blaylock, M.D. author of "Excitotoxins: The Taste That Kills." Excitotoxins are substances that when consumed in large amounts such as aspartame found in diet soda can trigger inflammatory and hyper-stimulated activity in the brain. The important thing to remember about all of this is you are not going to be able to avoid glutamate foods all together. The list is quite extensive, and various forms of glutamate-like substances are found in a wide variety of things we eat - many of them healthy. There is also going to be natural glutamate in a healthy diet because glutamate at a certain level is important for normal brain function. However, it is the artificial forms such as aspartame, MSG and others that are problematic. Here is the list: monosodium glutamate nutrasweet/aspartame malted barley flour glutamate hydrolyzed protein malt extract natural flavor(s) hydrolyzed vegetable protein (HVP) malt flavoring(s) natural flavorings(s) hydrolyzed plant protein malted barley/barley malt maltodextrin hydrolyzed oat flour malted anything carrageenan hydrolyzed anything textured protein gelatin sodium caseinate guar gum spice(s) calcium caseinate soy extract AutismRecoverySystem.com seasoning(s) caseinate soy protein seasoned salt disodium guanylate soy protein concentrate dough conditioner(s) disodium inosinate soy protein isolate yeast extract disodium caseinate soy sauce autolyzed yeast chicken/beef/pork "flavoring" whey protein autolyzed yeast extract chicken/beef/pork "base" whey protein isolate autolyzed anything bouillon whey protein concentrate broth vegetable gum kombu extract stock plant protein extract l-cysteine soup base smoke flavoring(s) ajinomoto AutismRecoverySystem.com The material contained within this document is not intended to replace the services and/or medical advice of a licensed health care practitioner, nor is it meant to encourage diagnosis and treatment of disease. -

Glutamate and Sodium Reduction
INTERNATIONAL GLUTAMATE TECHNICAL COMMITTEE Please Reply To IGTC: Avenue Jules Bordet 142, B-1140 Brussels, Belgium Tel: +81-80-3258-1900 FAX: +81-3-5250-8403 E-mail: [email protected] International Glutamate Technical Committee (IGTC) Avenue Jules Bordet 142 B-1140 Brussels, Belgium Glutamate Contributes to the EUROPE - PARIS European Committee for Umami Reduction of Dietary Sodium Intake (ECU) 153, rue de Courcelles F-75817 PARIS CEDEX 17, France The World Health Organization (WHO) recommendation on sodium JAPAN – TOKYO SOUTH KOREA-SEOUL Amino Acids Seasoning Alliance consumption for adults is 2 g sodium/day (equivalent to 5 g salt/day). However, of Northeast Asia (ASANA) most people consume much more with the current mean global sodium Hatchobori 3-11-8 Chuo-ku, Tokyo 104-0032, Japan consumption estimated to be at 3.95 g sodium/day (Mozaffarian et al. 2014). REPUBLIC OF CHINA - TAIPEI Taiwan Amino Acid Manufacturers Since high sodium intake is reported to be associated with various non- Association ROC (TAAMA) Yuan Hsing Bldg., 4th Floor. communicable diseases (NCDs) such as hypertension, cardiovascular disease No.6 Chang Chun Rd. Taipei, Taiwan, 104 and stroke, the reduction of sodium intake is a very important public health Republic of China concern around the world (WHO, 2003). SOUTH AMERICA - SAO PAULO Institute for Glutamate Sciences in South America (IGSSA) P.O. Box 5789 While sodium reduction in the diet is an important objective, when salt (NaCl) Sao Paulo SP, Brazil SOUTHEAST ASIA - BANGKOK levels are reduced in foods, its palatability is also generally decreased. Regional Committee for Glutamate Sciences (RCGS) Monosodium glutamate (MSG) is a flavour enhancer that contains about 12% 487/1 Si Ayutthaya Road Phaya Thai, Bangkok 10400 sodium, which is less than half of that contained in regular table salt at about Thailand 39%. -

Sodium Content and Food Additives in Major Brands of Brazilian Children's
DOI: 10.1590/1413-812320182312.21812016 4065 Sodium content and food additives in major brands ARTICLE ARTIGO of Brazilian children’s foods Conteúdo de sódio e aditivos alimentares das maiores marcas de alimentos destinados para crianças brasileiras Antônio Zenon Antunes Teixeira 1 Abstract The objective of this paper is to evaluate Resumo O objetivo deste trabalho é avaliar o the sodium content and additive usage in pack- teor de sódio e a utilização de aditivos em ali- aged food products targeted at Brazilian children. mentos industrializados destinados às crianças no Commercial database and manufactures’ websites Brasil. Bancos de dados comerciais foram usados were used to identify major brands of child-ori- para identificar as principais marcas de alimen- ented foods. Samples were categorized as “chil- tos. Foram classificadas como “alimentos para dren’s foods” (n = 214) and “infant’s foods” (n = crianças” (n = 214) e “alimentos infantis” (n = 86). Nutrition Facts labels were used to calculate 86). Os rótulos nutricionais foram usados para the median Na content (mg/serving, mg/kcal, and calcular a mediana de sódio (mg/porção, mg/kcal mg/100g); the ingredient lists to note the use of e mg/100g) e a lista de ingredientes comprovou o food additives. The sodium content found in sam- uso de aditivos alimentares. O teor de sódio en- ples varied widely among brands. The amount of contrado em amostras variou entre as marcas. A sodium was high (> 210 mg/serving) in 20% of quantidade de sódio foi elevada (> 210 mg/por- the products. Sodium density exceeded 1 mg/kcal ção) em 20% dos produtos. -

Umami and Salt Reduction
Hypertension Research (2020) 43:569–570 https://doi.org/10.1038/s41440-020-0414-4 COMMENT Umami and salt reduction Katsuyuki Ando1 Received: 23 January 2020 / Revised: 27 January 2020 / Accepted: 27 January 2020 / Published online: 19 February 2020 © The Japanese Society of Hypertension 2020 An umami substance L-glutamate was found in seaweed modifying effect on saltiness, might also not be affected by broth by the Japanese investigator Kikunae Ikeda in 1908 the condition of the human, although some patients with [1]. The term “umami” has been used worldwide since 1985 masked hypertension have an impairment of salty taste when the 1st International Symposium on Umami was held recognition [4]. In addition, the palatability in the 0.3% salt in Hawaii. Umami is one of five basic tastes, including plus MSG solution appeared to be slightly higher than that sweetness, sourness, bitterness, and saltiness, and is an in the 0.6 or 0.9% salt plus MSG solutions. The taste of independent taste unable to be made via combination of umami substance-added food may be more pleasant with other tastes. A receptor protein for umami [2], as well as for low salt levels than with high salt levels. sweetness and bitterness, was detected in taste cells on the The palatability of food is determined not only by taste 1234567890();,: 1234567890();,: tongue and palate epithelium, whereas ion channels for but also by visual, smelling, tactile, and auditory senses as sourness (H+) and saltiness (Na+) exist in taste cells. well as by dietary habits and the environment. Thus, some- Tasting umami flavors may be a unique sensory function to one might consider that the ameliorating action of umami on ameliorate the palatability of foods. -

Acceptable Daily Intake ADI.Pdf
REPORT FROM THE COMMISSION on Dietary Food Additive Intake in the European Union TABLE OF CONTENTS Executive Summary....................................................................................................................2 1 Introduction ..................................................................................................................3 2 Background...................................................................................................................4 3 The monitoring task......................................................................................................6 3.1 Additives excluded from the monitoring task and further examination:......................6 3.2 Additives subject to tier-1 screening ............................................................................6 3.3 Additives subject to tier-2 screening ............................................................................7 3.4 Additives subject to tier-3 screening ............................................................................7 4 The monitoring data......................................................................................................7 4.1 Instructions for reporting the monitoring data..............................................................7 4.2 The type of monitoring data obtained...........................................................................8 4.2.1 Age of data....................................................................................................................8 -
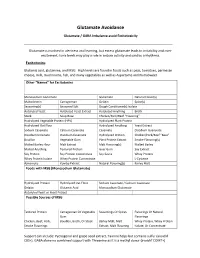
Glutamate Avoidance
Glutamate Avoidance Glutamate / GABA Imbalance avoid Excitotoxicity ____________________________________________________________________________ Glutamate is involved in alertness and learning, but excess glutamate leads to irritability and over- excitement; toxic levels may play a role in seizure activity and cardiac arrhythmia. Excitotoxins Glutamic acid, glutamine, and MSG. High levels are found in foods such as peas, tomatoes, parmesan cheese, milk, mushrooms, fish, and many vegetables as well as Aspartame and NutraSweet. Other “Names” for Excitotoxins Monosodium Glutamate Glutamate Natural Flavor(s) Maltodextrin Carrageenan Gelatin Spice(s) Seasoning(s) Seasoned Salt Dough Conditioner(s) Isolate Autolyzed Yeast Autolyzed Yeast Extract Autolyzed Anything Broth Stock Soup Base Chicken/Pork/Beef “Flavoring” Hydrolyzed Vegetable Protein (HPV) Hydrolyzed Plant Protein Hydrolyzed Oat Flour Hydrolyzed Anything Yeast Extract Sodium Caseinate Calcium Caseinate Caseinate Disodium Guanyiate Disodium Inosinate Disodium Caseinate Hydrolyzed Protein Chicken/Pork/Beef “Base” Bouillon Vegetable Gum Plant Protein Extract Smoke Flavoring(s) Malted Barley Flour Malt Extract Malt Flavoring(s) Malted Barley Malted Anything Textured Protein Guar Gum Soy Extract Soy Protein Soy Protein Concentrate Soy Sauce Whey Protein Whey Protein Isolate Whey Protein Concentrate L-Cysteine Ajinomoto Kombu Extract Natural Flavoring(s) Barley Malt Foods with MSG (Monosodium Glutamate) Hydrolyzed Protein Hydrolyzed Oat Flour Sodium Caseinate / Calcium Caseinate Gelatin -

Misleading and Deceptive Use of "No MSG" to Hide MSG
Misleading and deceptive use of "No MSG" to hide MSG http://www.truthinlabeling.org/nomsg.html WWW.truthinlabeling.org -- Home page Back to Table of Contents No MSG, No MSG, No MSG, No MSG, No MSG, No MSG, No MSG, No MSG, No MSG, No MSG, No MSG, No MSG, No MSG, No MSG MSG is sometimes hidden in food with labels that say "No Added MSG," "No MSG Added," and "No MSG" We found these a while back at Trader Joe's in Chicago: Imagine ORGANIC Free Range Chicken Broth Ingredients include "natural flavor" -- and Imagine doesn't tell what is in the "natural flavor" and/or provide an analysis of the amount of processed free glutamic acid in their product. Kitchen Basics Natural Chicken Stock Ingredients include "chicken stock," "natural flavor," and "vegetable stocks" -- and Kitchen Basics doesn't tell what is in the "chicken stock," "natural flavor," and "vegetable stock," and/or provide an analysis of the amount of processed free glutamic acid in their product. Manufacturers are acutely aware that many consumers would prefer not to have MSG (1) in their food.(2) Some manufacturers have responded by using "clean labels," i.e., labels that contain only ingredient names they think consumers will not recognize as containing MSG -- names such as "hydrolyzed soy protein." Others advertise "No MSG," "No MSG Added," or "No Added MSG," even though their products contain MSG. Most offenders are small processors who are possibly being misguided by the FDA, the USDA, and/or consultants. Hain and Campbell's, both large companies, are among those who have been alerted to both the deception that they are perpetrating and the illegality of what they are doing, yet continue with what the FDA has termed deceptive and misleading labeling. -

A Review on Synthetic and Natural Food Additives
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Biblioteca Digital do IPB Adding Molecules to Food, Pros and Cons – A Review on Synthetic and Natural Food Additives MÁRCIO CAROCHOa,b, MARIA FILOMENA BARREIROc, PATRICIA MORALESb,* and ISABEL C.F.R. FERREIRAa,* a Mountain Research Center (CIMO) ESA, Polytechnic Institute of Bragança, Campus de Santa Apolónia, Apartado 1172, 5301-855, Bragança, Portugal b Department of Nutrition and Bromatology II, Faculty of Pharmacy, Complutense University of Madrid, Pza Ramón y Cajal, s/n, E-28040 Madrid, Spain c Laboratory of Separation and Reaction Engineering (LSRE), Associate Laboratory LSRE/LCM, Polytechnic Institute of Bragança, Campus Santa Apolónia Apartado 1134, 5301-857 Bragança, Portugal *Corresponding authors: [email protected] (Tel: (34)913941802); [email protected] (Tel:(351)273303219 Fax: (351)273325405)) ABSTRACT The pressing issue to feed the increasing world population has created a demand to enhance food production, which has to be cheaper, but at the same time must meet high quality standards. Taste, appearance, texture, and microbiological safety are required to be preserved within a foodstuff for the longest period of time. Although considerable improvements have been achieved in terms of food additives, some are still enveloped in controversy. The lack of uniformity in worldwide laws regarding additives, along with conflicting results of many studies help foster this controversy. In this report the most important preservatives, nutritional additives, coloring, flavoring, texturizing, and miscellaneous agents are analyzed in terms of safety and toxicity. Natural additives and extracts, which are gaining interest due to changes in consumer habits are also evaluated in terms of their benefits to health and combined effects.