Concept Papers for Changes to Rule 9-1 -- Refinery Fuel Gas Sulfur
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Liquefied Petroleum Gas (LPG)
Liquefied Petroleum Gas (LPG) Demand, Supply and Future Perspectives for Sudan Synthesis report of a workshop held in Khartoum, 12-13 December 2010 The workshop was funded by UKaid from the Department for International Development Cover image: © UNAMID / Albert Gonzalez Farran This report is available online at: www.unep.org/sudan Disclaimer The material in this report does not necessarily represent the views of any of the organisations involved in the preparation and hosting of the workshop. It must be noted that some time has passed between the workshop and the dissemination of this report, during which some important changes have taken place, not least of which is the independence of South Sudan, a fact which greatly affects the national energy context. Critically, following the independence, the rate of deforestation in the Republic of Sudan has risen from 0.7% per year to 2.2% per year, making many of the discussions within this document all the more relevant. Whilst not directly affecting the production of LPG, which is largely derived from oil supplies north of the border with South Sudan, the wider context of the economics of the energy sector, and the economy as a whole, have changed. These changes are not reflected in this document. This being said, it is strongly asserted that this document still represents a useful contribution to the energy sector, particularly given its contribution to charting the breadth of perspectives on LPG in the Republic of Sudan. Liquefied Petroleum Gas (LPG) Demand, Supply and Future Perspectives for Sudan Synthesis report of a workshop held in Khartoum, 12-13 December 2010 A joint publication by: Ministry of Environment, Forestry and Physical Development – Sudan, Ministry of Petroleum – Sudan, United Kingdom Department for International Development, United Nations Development Programme and United Nations Environment Programme Table of contents Acronyms and abbreviations . -

CCS: Applications and Opportunities for the Oil and Gas Industry
Brief CCS: Applications and Opportunities for the Oil and Gas Industry Guloren Turan, General Manager, Advocacy and Communications May 2020 Contents 1. Introduction ................................................................................................................................... 2 2. Applications of CCS in the oil and gas industry ............................................................................. 2 3. Conclusion ..................................................................................................................................... 4 Page | 1 1. Introduction Production and consumption of oil and gas currently account for over half of global greenhouse gas emissions associated with energy use1 and so it is imperative that the oil and gas industry reduces its emissions to meet the net-zero ambition. At the same time, the industry has also been the source and catalyst of the leading innovations in clean energy, which includes carbon capture and storage (CCS). Indeed, as oil and gas companies are evolving their business models in the context of the energy transition, CCS has started to feature more prominently in their strategies and investments. CCS is versatile technology that can support the oil and gas industry’s low-carbon transition in several ways. Firstly, CCS is a key enabler of emission reductions in the industries’ operations, whether for compliance reasons, to meet self-imposed performance targets or to benefit from CO2 markets. Secondly, spurred by investor and ESG community sentiment, the industry is looking to reduce the carbon footprint of its products when used in industry, since about 90% of emissions associated with oil and gas come from the ultimate combustion of hydrocarbons – their scope 3 emissions. Finally, CCS can be a driver of new business lines, such as clean power generation and clean hydrogen production. From the perspective of the Paris Agreement, however, the deployment of CCS globally remains off track. -

Facts About Alberta's Oil Sands and Its Industry
Facts about Alberta’s oil sands and its industry CONTENTS Oil Sands Discovery Centre Facts 1 Oil Sands Overview 3 Alberta’s Vast Resource The biggest known oil reserve in the world! 5 Geology Why does Alberta have oil sands? 7 Oil Sands 8 The Basics of Bitumen 10 Oil Sands Pioneers 12 Mighty Mining Machines 15 Cyrus the Bucketwheel Excavator 1303 20 Surface Mining Extraction 22 Upgrading 25 Pipelines 29 Environmental Protection 32 In situ Technology 36 Glossary 40 Oil Sands Projects in the Athabasca Oil Sands 44 Oil Sands Resources 48 OIL SANDS DISCOVERY CENTRE www.oilsandsdiscovery.com OIL SANDS DISCOVERY CENTRE FACTS Official Name Oil Sands Discovery Centre Vision Sharing the Oil Sands Experience Architects Wayne H. Wright Architects Ltd. Owner Government of Alberta Minister The Honourable Lindsay Blackett Minister of Culture and Community Spirit Location 7 hectares, at the corner of MacKenzie Boulevard and Highway 63 in Fort McMurray, Alberta Building Size Approximately 27,000 square feet, or 2,300 square metres Estimated Cost 9 million dollars Construction December 1983 – December 1984 Opening Date September 6, 1985 Updated Exhibit Gallery opened in September 2002 Facilities Dr. Karl A. Clark Exhibit Hall, administrative area, children’s activity/education centre, Robert Fitzsimmons Theatre, mini theatre, gift shop, meeting rooms, reference room, public washrooms, outdoor J. Howard Pew Industrial Equipment Garden, and Cyrus Bucketwheel Exhibit. Staffing Supervisor, Head of Marketing and Programs, Senior Interpreter, two full-time Interpreters, administrative support, receptionists/ cashiers, seasonal interpreters, and volunteers. Associated Projects Bitumount Historic Site Programs Oil Extraction demonstrations, Quest for Energy movie, Paydirt film, Historic Abasand Walking Tour (summer), special events, self-guided tours of the Exhibit Hall. -

Refining Company Perspective & Approach to High Quality Binders
June 18, 2019 Refining company perspective & approach to high quality asphalts Pavel Kriz, Ph.D., P.Eng. Americas Asphalt Group & Discipline Technical Leader This communication may contain confidential information for the use of the entity to which it is addressed. If you are not the intended recipient, you are hereby notified that any use, dissemination, distribution, or copying of this communication is prohibited. If you are the intended recipient, information may not be reproduced or further distributed out of your organization without the originator's authorization in writing. 2019 © Imperial Oil Limited. All rights reserved. Imperial & ExxonMobil: Funding Members of Asphalt Institute 2 Global Asphalt Operations In NA Imperial & ExxonMobil manufacture asphalt at Joliet, Billings, Nanticoke & Strathcona Refineries • Produce over 2MTa of premium asphalt • Utilize high quality WestCan crudes • Committed to product quality & integrity Sarnia Technology Applications & Research • Canada’s first & largest petroleum research est. 1924 • 700+ patents • ~40+ PhDs out of ~100 employees • Global Centre of Excellence for Asphalt Asphalt Production • Long heritage & deep expertise in asphalt research Asphalt Technology/R&D 3 Crude Oil • “Crude oil is a naturally occurring, yellowish-black liquid found in geological formations beneath the Earth's surface” • It is a mixture of a large number of different hydrocarbons • Each oil has a unique mix of molecules, which define its properties, like color and viscosity Composition by weight Element Percent range Carbon 83 to 85% Hydrogen 10 to 14% Nitrogen 0.1 to 2% Oxygen 0.05 to 1.5% Sulphur 0.05 to 6.0% Metals < 0.1% 4 Crude Oil Origin 5 Crude Oil Reservoir Saturated porous reservoir rock Impermeable layers (e.g. -

Fire from Ice
SYSTEM FAILURE CASE STUDY NOVEMBER 2009 VOLUME 3 ISSUE 08 Fire From Ice February 16, 2007: Propane gas leaked from the McKee Refinery’s Propane Deasphalting Unit in Sunray, Texas. As winds carried the vapor cloud, a spark ignited the propane and the entire cloud burst into flames. The fire spread quickly, forcing an evacuation of the refinery as firefighters battled the blaze. Three workers suffered serious burns and the refinery was shut down for two months. Gas prices increased 9 cents per gallon in the west. BACKGROUND Industrial Overview hamrock Oil and Gas built the McKee Refinery outside Sunray, Texas in 1933. The refinery Sunderwent major modifications in the 1950s and 1990s. Valero acquired the refinery in 2001 and further Figure 1: The Refinery Fire upgraded it in 2004. By 2005, Valero was the largest refiner in North America, with eighteen refineries producing 3.3 million barrels per day. The McKee Refinery contributed WHAT HAPPENED? about 170,000 barrels per day to Valero’s overall production. In February 2007, a four-day cold front froze the water in the PROPANE DEASPHALTING (PDA) UNIT dead-leg pipe, causing it to expand, and crack the pipe. Ice One part of the refinery is the Propane Deasphalting (PDA) blocked the crack and prevented any propane from exiting. Unit, where high-pressure propane serves as a solvent to recover fuel and asphalt from crude oil residues. In 1992, February 16, 2007: Freezing water cracked an the refinery shut down a control station in the PDA unit. idle section of pipe causing a propane gas Rather than remove the idle subsection, or isolate it with slip 1 release. -

Refinery Processing of Asphalt Cement
Transportation Research Record 999 1 Refinery Processing of Asphalt Cement LUKE W. CORBETT ABSTRACT BOSCAN ARABIAN NIGERIA VENEZUELA HEAVY ___h!Qfil_ API Degrees ~ ~ 38.1 SP. Gravity .999 .886 .834 Crude petroleum varies in its makeup of dis % Sulfur 6-4 2.8 0.2 tillable fractions as well as in its bitumen content. Initially, it is separated by dis tillation with the major volume of asphalt Gasoline cement manufactured today prepared by Keroslne 1--~-<-.... straight reduction to grade. Other process Lt. Gas Oil ,___ .,. .,_ ing methods, such as solvent deasphalting, supercritical extraction, blending, and con Hv ~ Gas OHS tinuous air-blowing, supply fractions that are usable in asphalt cement within the limitations of specification compliance. Bitumen Crude oils may be arbitrarily classified Residuum according to their API (American Petroleum Institute) gravity, which is shown to relate to composition, physical properties, and the viscosity-penetration relationship of the asphalt cement therefrom. It is also related FIGURE 1 Make-up of crude petroleum. to distillation cut-points and how that crude might best be processed. The viscos ity-penetration relationship is shown to distillable overhead fractions with a high percent correlate with temperature susceptibility age of bitumen, whereas the high API gravity crudes and may be used directly in determining contain a relatively high percentage of overhead specification compliance. The viscosity fraction with a low percentage of bitumen. The low graded (Table 2) specifications as used in gravity crudes are generally referred to as heavy the United States are more rational than the crudes or as sour crudes if their sulfur content is penetration-softening point control systems high. -
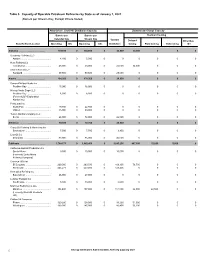
Table 3. Capacity of Operable Petroleum Refineries by State As of January 1, 2021 (Barrels Per Stream Day, Except Where Noted)
Table 3. Capacity of Operable Petroleum Refineries by State as of January 1, 2021 (Barrels per Stream Day, Except Where Noted) Atmospheric Crude Oil Distillation Capacity Downstream Charge Capacity Barrels per Barrels per Thermal Cracking Calendar Day Stream Day Vacuum Delayed Other/Gas State/Refiner/Location Operating Idle Operating Idle Distillation Coking Fluid Coking Visbreaking Oil Alabama......................................................... 139,600 0 145,600 0 54,000 34,000 0 0 0 Goodway Refining LLC ....................................................................................................................................................................................................Atmore 4,100 0 5,000 0 0 0 0 0 0 Hunt Refining Co ....................................................................................................................................................................................................Tuscaloosa 48,000 0 50,000 0 25,000 34,000 0 0 0 Shell Chemical LP ....................................................................................................................................................................................................Saraland 87,500 0 90,600 0 29,000 0 0 0 0 Alaska......................................................... 164,200 0 178,500 0 26,000 0 0 0 0 ConocoPhillips Alaska Inc ....................................................................................................................................................................................................Prudhoe -

5.1 Petroleum Refining1
5.1 Petroleum Refining1 5.1.1 General Description The petroleum refining industry converts crude oil into more than 2500 refined products, including liquefied petroleum gas, gasoline, kerosene, aviation fuel, diesel fuel, fuel oils, lubricating oils, and feedstocks for the petrochemical industry. Petroleum refinery activities start with receipt of crude for storage at the refinery, include all petroleum handling and refining operations, and they terminate with storage preparatory to shipping the refined products from the refinery. The petroleum refining industry employs a wide variety of processes. A refinery’s processing flow scheme is largely determined by the composition of the crude oil feedstock and the chosen slate of petroleum products. The example refinery flow scheme presented in Figure 5.1-1 shows the general processing arrangement used by refineries in the United States for major refinery processes. The arrangement of these processes will vary among refineries, and few, if any, employ all of these processes. Petroleum refining processes having direct emission sources are presented on the figure in bold-line boxes. Listed below are 5 categories of general refinery processes and associated operations: 1. Separation processes a. Atmospheric distillation b. Vacuum distillation c. Light ends recovery (gas processing) 2. Petroleum conversion processes a. Cracking (thermal and catalytic) b. Reforming c. Alkylation d. Polymerization e. Isomerization f. Coking g. Visbreaking 3. Petroleum treating processes a. Hydrodesulfurization b. Hydrotreating c. Chemical sweetening d. Acid gas removal e. Deasphalting 4. Feedstock and product handling a. Storage b. Blending c. Loading d. Unloading 5. Auxiliary facilities a. Boilers b. Waste water treatment c. Hydrogen production d. -

Sources of Propane Consumed in California
ANL-20/73 Sources of Propane Consumed in California Energy Systems Division About Argonne National Laboratory Argonne is a U.S. Department of Energy laboratory managed by U Chicago Argonne, LLC under contract DE-AC02-06CH11357. The Laboratory’s main facility is outside Chicago, at 9700 South Cass Avenue, Argonne, Illinois 60439. For information about Argonne and its pioneering science and technology programs, see www.anl.gov. DOCUMENT AVAILABILITY Online Access: U.S. Department of Energy (DOE) reports produced after 1991 and a growing number of pre-1991 documents are available free at OSTI.GOV (http://www.osti.gov/), a service of the US Dept. of Energy’s Office of Scientific and Technical Information. Reports not in digital format may be purchased by the public from the National Technical Information Service (NTIS): U.S. Department of Commerce National Technical Information Service 5301 Shawnee Rd Alexandria, VA 22312 www.ntis.gov Phone: (800) 553-NTIS (6847) or (703) 605-6000 Fax: (703) 605-6900 Email: [email protected] Reports not in digital format are available to DOE and DOE contractors from the Office of Scientific and Technical Information (OSTI): U.S. Department of Energy Office of Scientific and Technical Information P. O. B o x 6 2 Oak Ridge, TN 37831-0062 www.osti.gov Phone: (865) 576-8401 Fax: (865) 576-5728 Email: [email protected] Disclaimer This report was prepared as an account of work sponsored by the Western Propane Gas Association. Neither the United States Government nor any agency thereof, nor U Chicago Argonne, LLC, nor any of their employees or officers, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. -

Tar Sands Crude Oil - Health Effects of a Dirty and Destructive Fuel (PDF)
NRDC: Tar Sands Crude Oil - Health Effects of a Dirty and Destructive Fuel (PDF) FEBRUARY 2014 NRDC ISSUE BRIEF IB:14-02-B Tar Sands Crude Oil: Health Effects of a Dirty and Destructive Fuel S yncrude upgrader, Fort McMurray © Julia Kilpatrick, Pembina yncrude upgrader, Three recent studies confirm that tar sands processing near Fort McMurray and Edmonton, Alberta is resulting in the release of cancer-causing chemicals. I nstitute he boreal forest in Northern Canada lies atop massive tar sands deposits that are roughly the size of Florida. Crude oil produced from these tar sands is one of the world’s dirtiest and most environmentally Tdestructive sources of fuel. Today, energy companies in Canada are actively developing this dirty fuel and have plans to triple production in the coming years. As tar sands is strip-mined and drilled from beneath the boreal forest of Alberta, Canada, greater volumes of tar sands–derived crude oil will be transported through North America’s communities. With more tar sands flowing through pipelines, moving on railcars, and being processed at refineries, there is mounting evidence that people and communities in the vicinity of tar sands activity face substantial health and safety risks. This report highlights a growing body of scientific research, and news reports about people directly impacted, showing that serious health risks and problems are arising all along this tar sands network, from northern Canada to refineries in California, the Gulf Coast, the Midwest and the Rocky Mountains, as well as from accidents and spills. Despite this deepening danger, to date, state and federal governments have done too little to address this threat to the public’s health. -

Equipment Design and Cost Estimation for Small Modular Biomass Systems, Synthesis Gas Cleanup and Oxygen Separation Equipment”
A national laboratory of the U.S. Department of Energy Office of Energy Efficiency & Renewable Energy National Renewable Energy Laboratory Innovation for Our Energy Future Equipment Design and Cost Subcontract Report NREL/SR-510-39943 Estimation for Small Modular May 2006 Biomass Systems, Synthesis Gas Cleanup, and Oxygen Separation Equipment Task 1: Cost Estimates of Small Modular Systems Nexant Inc. San Francisco, California NREL is operated by Midwest Research Institute ● Battelle Contract No. DE-AC36-99-GO10337 Equipment Design and Cost Subcontract Report NREL/SR-510-39943 Estimation for Small Modular May 2006 Biomass Systems, Synthesis Gas Cleanup, and Oxygen Separation Equipment Task 1: Cost Estimates of Small Modular Systems Nexant Inc. San Francisco, California NREL Technical Monitor: Kelly Ibsen Prepared under Subcontract No. ACO-5-44027 National Renewable Energy Laboratory 1617 Cole Boulevard, Golden, Colorado 80401-3393 303-275-3000 • www.nrel.gov Operated for the U.S. Department of Energy Office of Energy Efficiency and Renewable Energy by Midwest Research Institute • Battelle Contract No. DE-AC36-99-GO10337 This publication was reproduced from the best available copy Submitted by the subcontractor and received no editorial review at NREL NOTICE This report was prepared as an account of work sponsored by an agency of the United States government. Neither the United States government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. -

From the Field to the Table
From the to the Field Table A Comparison of Beet Sugar Processing and Cane Sugar Refining SUGAR BEET PROCESSING FACTORY ugar comes from sugarcaneFILTRA andTION sugarFIL beets,TRATION but how does it get out of the field and onto the SUGAR BEET CENTRIFUGALS FOOD MANUFACTURER SLICING STORAGE Stable? Fortunately, nature has taken care of making the sugar; the cane and beets doBULK that. We LIQUID just have to extract andCARBONA purifyTION the sugar (sucrose) from these plants. In Louisiana sugarcane WASHING DIFFUSION CRYSTALLIZATION BAGS DRYERS SCREENS mills and refineries are the next stepEVAPORA onceTORS the cane IN VACCUM leaves PANS the field. CONSUMER CANE SUGAR REFINERY SUGARCANE EVAPORATORS FILTRATION CENTRIFUGALS CRUSHING CLARIFICATION RAW SUGAR MILL VACCUM PANS CENTRIFUGALS FILTRATION EVAPORATORS CENTRIFUGALS FOOD MANUFACTURER STORAGE BULK MINGLER LIQUID MELTER CHARCOAL RAW SUGAR WAREHOUSE FILTRATION CRYSTALLIZATION BAGS COLUMNS DRYERS SCREENS IN VACCUM PANS CONSUMER CANE GROWTH & HARVEST In Louisiana, sugarcane begins to grow very fast after the last threat of a freeze is over, but cane grows fastest during the hot summer months. Usually by late September, the cane is ready to harvest. Large machines called combine harvesters cut the standing cane into pieces called billets and loads the billets into wagons and trailers. The cane is brought to the sugar mill for grinding. SUGARCANE MILLS After it’s harvested, the sugarcane goes to a mill located near the field, where the raw sugar 2 Evaporation — The sugar juice is filtered. is separated from the plant and shipped to the Then the juice is boiled to remove the water refinery. Here’s how it works: At the mill, the in a process called evaporation.