Supporting Information
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Baden-Württemberg Exchange Program
Baden-Württemberg Exchange Program Program Overview This program is a North Carolina Exchange program hosted by UNC Greensboro. In this unique program, North Carolina students have the chance to study at one of the Baden-Wuerttemberg Universities in Germany, and in exchange, Baden-Wuerttemberg students have the opportunity to study at one of the participating North Carolina public institutions. Program Facts Application & Eligibility Locations Program Dates *University of Mannheim (Mannheim) (Karlsruhe, Konstanz, Tübingen, and Hohenheim ) Heidelberg University (Heidelberg) Full Academic Year .................... Aug, Sept, or Oct to July *University of Hohenheim (Stuttgart) Spring .........................................Jan, Feb, or April to July *Karlsruhe Institute of Technology (KIT) (Karlsruhe) *University of Konstanz (Konstanz) Application Deadlines University of Stuttgart (Stuttgart) Fall/Academic Year ...................................... Mid-February *University of Tübingen (Tübingen) Spring ......................................................... Early October University of Ulm (Ulm) University of Freiburg *spring options Eligibility • (All but Mannheim) Minimum equivalency of two years of German Type of Program ............................................... Exchange • (Mannheim) Two years of German if taking German Program Dates classes • Must a degree-seeking student (Most Locations) • Have at least sophomore standing Full Academic Year ........................ October to September • Have at least a 2.75 cumulative GPA Spring -

Inaugural Event for “Excellent“ Energy Research
Press Release No. 044 | mhe | march 26, 2019 Inaugural Event for “Excellent“ Energy Research Official Opening of the CELEST Research Platform and POLiS Battery Cluster of Excellence 1. Bildunterschrift ( max. zweizeilig + Zeil e für Quellenangabe Launch of the Center for Electrochemical Energy Storage Ulm & Karlsruhe (CELEST), one of the biggest German research and development platforms in the area of electrochemical energy storage. Together with guests from politics, research, and indus- try, KIT, Ulm University, and the Center for Solar Energy and Hy- KIT Energy Center: Having future in mind drogen Research Baden-Württemberg (ZSW) celebrated the opening of the joint research platform and its first outstanding Monika Landgraf success: approval of the Cluster of Excellence on Post Lithium Chief Press Officer, Storage (POLiS) within the Excellence Strategy launched by the Head of Corp. Communications federal and state governments. Germany's largest electrochemical energy research platform was of- Kaiserstraße 12 ficially launched today, Tuesday, March 26: At the Center for Electro- 76131 Karlsruhe chemical Energy Storage Ulm & Karlsruhe (CELEST), researchers Phone: +49 721 608-21105 from various disciplines are developing high-performance and envi- Email: [email protected] ronmentally friendly energy storage systems – which are urgently Press contact: needed for a successful energy revolution and climate-friendly elec- tric mobility. The platform was co-founded by the Karlsruhe Institute Helmholtz Institute Ulm (HIU): of Technology (KIT), Ulm University, and the Center for Solar Energy Daniel Messling and Hydrogen Research Baden-Württemberg (ZSW). State Secretary Phone: +49 731 50 34013 of the Federal Ministry of Education and Research (BMBF), Christian [email protected] Luft, and Ministerial Director of the Ministry of Science, Research and the Arts Baden-Württemberg, Ulrich Steinbach, attended the inaugu- Karlsruhe Institute of Technology (KIT): ration ceremony at the Helmholtz Institute Ulm to honor the platform's Dr. -

Geometric Proportioning Strategies in Gothic Architectural Design Robert Bork*
$UFKLWHFWXUDO Bork, R 2014 Dynamic Unfolding and the Conventions of Procedure: Geometric +LVWRULHV Proportioning Strategies in Gothic Architectural Design. Architectural Histories, 2(1): 14, pp. 1-20, DOI: http://dx.doi.org/10.5334/ah.bq RESEARCH ARTICLE Dynamic Unfolding and the Conventions of Procedure: Geometric Proportioning Strategies in Gothic Architectural Design Robert Bork* This essay explores the proportioning strategies used by Gothic architects. It argues that Gothic design practice involved conventions of procedure, governing the dynamic unfolding of successive geometrical steps. Because this procedure proves difficult to capture in words, and because it produces forms with a qualitatively different kind of architectural order than the more familiar conventions of classical design, which govern the proportions of the final building rather than the logic of the steps used in creating it, Gothic design practice has been widely misunderstood since the Renaissance. Although some authors in the nineteenth and twentieth centuries attempted to sympathetically explain Gothic geometry, much of this work has been dismissed as unreliable, especially in the influential work of Konrad Hecht. This essay seeks to put the study of Gothic proportion onto a new and firmer foundation, by using computer-aided design software to analyze the geometry of carefully measured buildings and original design drawings. Examples under consideration include the parish church towers of Ulm and Freiburg, and the cross sections of the cathedrals of Reims, Prague, and Clermont-Ferrand, and of the Cistercian church at Altenberg. Introduction of historic monuments to be studied with new rigor. It is Discussion of proportion has a curiously vexed status in finally becoming possible, therefore, to speak with reason- the literature on Gothic architecture. -

Airport Stuttgart 1. Take the S-Bahn Line S2 Or S3 Towards Hauptbahnhof Stuttgart the Trains Leave Frequently All 10 Respectively 20 Minutes
Train directions from airport Stuttgart to BPM 09 at Ulm University expected duration: about 2 hours start: airport Stuttgart 1. Take the S-Bahn line S2 or S3 towards Hauptbahnhof Stuttgart the trains leave frequently all 10 respectively 20 minutes. 27 mins 2. Take a train towards Ulm Hauptbahnhof the fastest way: ICE trains 1 hour the cheapest way: regional trains (ask at the desk for a Baden-Württemberg ticket) 1 h 20 mins 3. Leave the train station of Ulm and walk straight ahead Now you have either the possibility to take a taxi -> 4a or you can take the bus to university -> 4b 4a. Taxis can normally be found close to the train station, if none is available you can call the service hotline (tel.: +49 731 / 66066) and ask for one. Ask the driver to go to James-Franck-Ring at the university, the BPM’09 is located in the O27 building. 10 mins 4b. The bus station is located between the two lanes of the Friedrich-Ebert-Straße, which is the road in front of the train station. You find the buses running towards university on the opposite side of the road. Lines 3 and 6 are running frequently towards University Süd. Leave the bus at the station called “Universität Süd” from where you can see a number of buildings. Now follow the sidewalk to the rightmost building you can see. 10 mins destination: the BPM’09 is located in the O27 building For further information and ticket reservation please visit the following websites: airport Stuttgart -> http://www.stuttgart-airport.com/sys/index.php?section_id=0&id=0&lang=1 Stuttgart main train station -> http://www.stgt.com/stuttgart/homee.htm Die Bahn (german railway service) -> http://www.bahn.de/international/view/en/index.shtml P P P to Uni West P Rectorate James-Franck-Ring Albert-Einstein-Allee P Deanery Ulm University P building ground entrance north of the new N27 surgery BPM’09 O29 entrance clinical O27 O28 centre entrance cafeteria south Clinical Centre Universität Süd Caption of the map street track your route bus station to downtown by car your route P parking space by bus O27 change street building . -

Sciendo · Docendo · Curando Ulm University Magazine
Nr. 01 I January 2016 Sciendo · Docendo · Curando Ulm University Magazine Treating trauma: Doctors and scientists work hand in hand Page 4 The unravelling of DNA architecture Page 12 Studying smartphone addiction in the wet lab Page 38 Alumni Homecoming: Back to the alma mater Page 44 2 | Preface euros and we have established numerous coop- erations with internationally renowned research institutions as well as with companies in the Science City Ulm and elsewhere. What is more, a publication analysis by Thomson Reuters has revealed that three of the world’s most influen- tial scientists in their fields carry out research at Ulm University. Photo: Eberhardt/Ulm University Eberhardt/Ulm Photo: By now, more than 10,000 young people pursue their degrees in our Faculties: Natural Sciences, Mathematics and Economics, the Medical Fac- ulty and the Faculty of Engineering, Computer Science and Psychology. More than ten per cent of our students have an international back- ground. They are predominantly enrolled in Eng- lish-taught master’s programmes or they opt for a PhD in the International Graduate School in Molecular Medicine, which is funded by the Ger- man ’Excellence Initiative’. Over the next semes- ters we will happily welcome refugees from crisis regions all over the world to our University and to the city of Ulm, birthplace of Nobel laureate Albert Einstein. I was elected President of Ulm University only a few months ago and look forward to shaping its Dear Readers, future. Thanks to my predecessors, President You have just opened the first issue of Ulm Uni- Professor Karl Joachim Ebeling and his University versity’s English-language magazine. -

2020 07 Broschuere Forschen
RESEARCH TOPICS IN DETAIL The airtight containers contain colourful punching plates. as charged particles. However, the internal processes are They are made from magnesium, calcium or sodium ele- complex. “Even experts like us have not yet fully figured ments that could play an important role in the batteries out what exactly is happening inside”, says Ehrenberg. of the future. Here in the lab, they are tested for suitabili- ty. “It is one of many areas we work in”, says Ulm-based This is also true of the well-known lithium ion technology. Energy Professor Dr. Maximilian Fichtner, Scientific Spokesperson Although the capacity and number of charging cycles have for CELEST, Europe’s largest research platform for electro- now increased to a high level and the costs are reasona- chemical energy storage. ble, scientists are puzzled for example by what is referred to as an ‘interfacial layer’ that develops in the lithium ion Since 2018, CELEST has pooled the expertise of three battery when a small quantity of the electrolyte corrodes CELEST major research institutions in Baden-Württemberg. They on an electrode. Researchers from CELEST are working to are Karlsruhe Institute of Technology (KIT), Ulm University understand this phenomenon, which determines battery and the Center for Solar Energy and Hydrogen Research quality and is also important for service life. (ZSW) in Ulm, which also operates Europe’s largest pi- lot plant for battery cell manufacture. These partners also Even though lithium ion technology is in use around the work together in the Post Lithium Storage (POLiS) Clus- globe, it does have disadvantages, as one of its main com- ter of Excellence, which has a significant influence on ponents is still cobalt. -
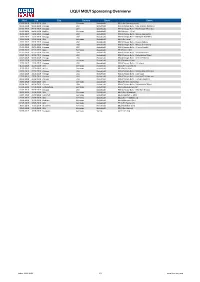
LIQUI MOLY Sponsoring Overvierw
LIQUI MOLY Sponsoring Overvierw Start End City Country Sport Event 02.02.2019 02.02.2019 Ulm Germany Basketball BBL (Ulm-Bremerhaven) 06.02.2019 06.02.2019 Chicago USA Basketball NBA (Chicago Bulls - New Orleans Pelicans) 09.02.2019 09.02.2019 Chicago USA Basketball NBA (Chicago Bulls - Washington Wizards) 09.02.2019 09.02.2019 Gießen Germany Basketball BBL (Giessen - Ulm) 11.02.2019 11.02.2019 Chicago USA Basketball NBA (Chicago Bulls - Milwaukee Bucks) 13.02.2019 13.02.2019 Chicago USA Basketball NBA (Chicago Bulls - Memphis Grizzlies) 13.02.2019 13.02.2019 Ulm Germany Basketball BBL (Ulm-Jena) 23.02.2019 23.02.2019 Chicago USA Basketball NBA (Chicago Bulls - Boston Celtics) 25.02.2019 25.02.2019 Chicago USA Basketball NBA (Chicago Bulls - Milwaukee Bucks) 01.03.2019 01.03.2019 Chicago USA Basketball NBA (Chicago Bulls - Atlanta Hawks) 02.03.2019 02.03.2019 Bonn Germany Basketball BBL (Bonn-Ulm) 05.03.2019 05.03.2019 Chicago USA Basketball NBA (Chicago Bulls - Indiana Pacers) 06.03.2019 06.03.2019 Chicago USA Basketball NBA (Chicago Bulls - Philadelphia 76ers) 08.03.2019 08.03.2019 Chicago USA Basketball NBA (Chicago Bulls - Detroit Pistons) 10.03.2019 10.03.2019 Bamberg Germany Basketball BBL (Bamberg-Ulm) 12.03.2019 12.03.2019 Chicago USA Basketball NBA (Chicago Bulls - LA Lakers) 16.03.2019 16.03.2019 Ulm Germany Basketball BBL (Ulm-Crailsheim) 19.03.2019 19.03.2019 Berlin Germany Basketball BBL (Berlin-Ulm) 20.03.2019 20.03.2019 Chicago USA Basketball NBA (Chicago Bulls - Washington Wizards) 23.03.2019 23.03.2019 Chicago USA Basketball -

Expeditionplus! Bicycling the Danube River from Germany's Black Forest to Romania's Black Sea Coast
Overview Bicycle Tours in Germany: ExpeditionPlus! Bicycling the Danube River from Germany's Black Forest to Romania's Black Sea Coast OVERVIEW This bicycle expedition combines two bicycle tours that travel the full length of the Danube River from Germany's Black Forest to the delta of the Danube in Romania on the Black Sea coast. We bicycle through some of the great capitals of central and eastern Europe on this bicycle ride through history, including Vienna, Budapest, Belgrade and Bucharest, Romania. During the first two weeks our bike ride follows the border of classical Rome in Germany. We follow some of the most important Medieval trade routes from Southern Germany into the Alps and visit historic Medieval towns such as Ulm, Regensburg, and Linz. For anybody who can't take time off for the whole thing, we'll split this tour in two for you so you can do just the first fifteen days from the Black Forest to Budapest, Hungary , or you can do the last two and a half weeks from Budapest to the Black Sea. HIGHLIGHTS Regensburg, Vienna, Budapest, Black, Forest Ulm, Belgrade, Bucharest and the Black Sea TOUR FACTS Tour Style : Learn more about our tours at https://www.experienceplus.com/tours/bike-tour-styles/-tours 33 days, 32 nights accommodation, 22 dinners (excluding drinks), all breakfasts, dinner cruise in Includes Budapest Countries Austria, Bulgaria, Croatia, Germany, Hungary, Romania, Slovakia, Serbia Begin/End Donaueschingen, Germany/Bucharest, Romania Arrive/Depart Zurich or Frankfurt, Germany/Bucharest, Romania Total Distance About 2880 km (1790 miles) Avg. Daily Distance 55 - 169 km (34 - 105 miles) per riding day Tour Level We work hard to maintain consistency across all of our tours, but some trips have unique differences. -

The Geography of Germany: Lessons for Teaching the Five Themes of Geography
DOCUMENT RESUME ED 460 910 SO 029 411 AUTHOR Blankenship, Glen; Tinkler, D. William TITLE The Geography of Germany: Lessons for Teaching the Five Themes of Geography. Social Studies, Grades 9-12. Update 1997/1998. INSTITUTION Inter Nationes, Bonn (Germany).; Goethe House, New York, NY. PUB DATE 1998-00-00 NOTE 105p.; Transparency 7 is not available from ERIC. For earlier version, see ED 396 972. AVAILABLE FROM National Council for the Social Studies, NCSS Publications, P.O. Box 2067, Waldorf, 'MD 20604-2067. Tel: 800-683-0812 (Toll Free); Fax: 301-843-0159; e-mail: [email protected]; Web site: http://www.ncss.org/home/ncss. PUB TYPE Guides Classroom Teacher (052) EDRS PRICE MF01/PC05 Plus Postage. DESCRIPTORS Area Studies; Civics; Cultural Education; Culture; Foreign Countries; *Geographic Concepts; *Geographic Location; *Geographic Regions; Geography; *Geography Instruction; High Schools; *Human Geography; Maps; Multicultural Education; Physical Geography; Social Studies; World Geography; World History IDENTIFIERS *Germany ABSTRACT This packet contains five lessons related to the five themes of geography: location; place; human-environment interaction; movement; and region. The lessons are designed to support the teaching of courses in world geography, U.S. government/civics, and economics from a comparative U.S./German perspective. Lessons include:(1) "Location of Germany on the Earth's Surface"; (2) "Physical and Human Characteristics of Germany"; (3) "The Interaction of the German People and Their Environment";(4) "Cultural Diversity in -

Typically Asymptomatic but with Robust Antibody Formation: Children's Unique Humoral Immune Response to SARS-Cov-2
medRxiv preprint doi: https://doi.org/10.1101/2021.07.20.21260863; this version posted July 22, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. All rights reserved. No reuse allowed without permission. 1 Typically asymptomatic but with robust antibody formation: Children’s unique 2 humoral immune response to SARS-CoV-2 3 Hanna Renk MD1*, Alex Dulovic Dr.rer.nat2*, Matthias Becker M.Sc2, Dorit Fabricius MD3, Maria Zernickel3, Daniel 4 Junker M.Sc2, Alina Seidel M.Sc4, Rüdiger Groß M.Sc4, Alexander Hilger M.Sc5, Sebastian Bode MD3, Linus 5 Fritsch5, Pauline Frieh5, Anneke Haddad DPhil5, Tessa Görne5, Jonathan Remppis MD1, Tina Ganzemueller MD7, 6 Andrea Dietz Dr.biol.hum6, Daniela Huzly MD8, Hartmut Hengel MD8, Klaus Kaier PhD9, Susanne Weber PhD9, 7 Eva-Maria Jacobsen PhD3, Philipp D. Kaiser Dr.rer.nat2, Bjoern Traenkle Dr.rer.nat2, Ulrich Rothbauer Dr.rer.nat2, 8 Maximilian Stich MD10, Burkhard Tönshoff10, Georg F. Hoffmann10, Barbara Müller PhD10,11, Carolin Ludwig12,13,14, 9 Bernd Jahrsdörfer MD12,13,14, Hubert Schrezenmeier MD12,13,14, Andreas Peter MD15, Sebastian Hörber MD15, 10 Thomas Iftner PhD7, Jan Münch PhD4, Thomas Stamminger MD6, Hans-Jürgen Groß MD16, Martin Wolkewitz 11 PhD9, Corinna Engel Dr.biol.hum1,17, Marta Rizzi MD18, Philipp Henneke MD5,19, Axel R. Franz MD1,17, Klaus- 12 Michael Debatin MD3, Nicole Schneiderhan-Marra Dr.rer.nat2, Ales Janda MD3,# and Roland Elling MD5,19,#,† 13 1 – University -
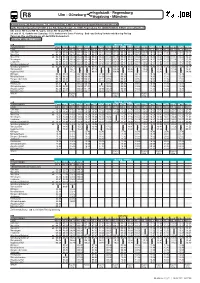
Re9|Re18|Rb15
Ingolstadt - Regensburg R8 Ulm - Günzburg Augsburg - München Kundendialog DB Regio Bayern, Tel. (089) 20355000, E-Mail: [email protected] agilis Eisenbahngesellschaft mbH & Co. KG, Galgenbergstr. 2a, 93053 Regensburg, Tel. (0800) 5892840, E-Mail: [email protected] DB: Linien RE 9 und RB 78 / agilis: Linien RE 18 und RB 15 24. und 31.12. Verkehr wie Samstag - 15.8. Verkehr wie Sonn-/Feiertag - Buß- und Bettag Verkehr wie Montag-Freitag Zwischen Ulm und Nersingen gilt der DING-Verbundtarif. Fahrplan gültig ab 13.12.2020 Montag - Freitag ZUGNUMMER 57007 57009 84233 57111 57113 84239 57115 57017 84245 57121 84249 57025 84255 57129 84257 57033 84259 57137 84263 LINIE RE 9 RE 9 RB 15 RE 9 RE 9 RB 15 RE 9 RE 9 RB 15 RE 9 RB 15 RE 9 RB 15 RE 9 RB 15 RE 9 RB 15 RE 9 RB 15 Ulm Hbf 04.46 05.22 05.27 05.45 06.21 06.25 06.43 07.23 07.45 08.23 08.48 09.23 09.48 10.23 10.48 11.23 11.48 12.23 12.48 Neu-Ulm an 04.49 05.24 05.30 05.47 06.24 06.29 06.46 07.26 07.49 08.26 08.51 09.26 09.51 10.26 10.51 11.26 11.51 12.26 12.51 Neu-Ulm ab 04.49 05.25 05.30 05.48 06.24 06.29 06.46 07.26 07.49 08.26 08.51 09.26 09.51 10.26 10.51 11.26 11.51 12.26 12.51 Nersingen 04.54 05.30 05.36 05.53 06.30 06.35 06.51 07.32 07.55 08.32 08.56 09.32 09.56 10.32 10.56 11.32 11.56 12.32 12.56 Leipheim 04.59 05.35 05.40 05.59 06.35 06.40 06.56 07.37 08.00 08.37 09.01 09.36 10.01 10.37 11.01 11.37 12.01 12.37 13.01 Günzburg Bahnhof an 05.03 05.39 05.44 06.04 06.39 06.44 07.01 07.41 08.03 08.41 09.06 09.41 10.05 10.41 11.06 11.41 12.05 12.41 13.06 Günzburg Bahnhof -

Pioneers of Modern Geography: Translations Pertaining to German Geographers of the Late Nineteenth and Early Twentieth Centuries Robert C
Wilfrid Laurier University Scholars Commons @ Laurier GreyPlace 1990 Pioneers of Modern Geography: Translations Pertaining to German Geographers of the Late Nineteenth and Early Twentieth Centuries Robert C. West Follow this and additional works at: https://scholars.wlu.ca/grey Part of the Earth Sciences Commons, and the Human Geography Commons Recommended Citation West, Robert C. (1990). Pioneers of Modern Geography: Translations Pertaining to German Geographers of the Late Nineteenth and Early Twentieth Centuries. Baton Rouge: Department of Geography & Anthropology, Louisiana State University. Geoscience and Man, Volume 28. This Book is brought to you for free and open access by Scholars Commons @ Laurier. It has been accepted for inclusion in GreyPlace by an authorized administrator of Scholars Commons @ Laurier. For more information, please contact [email protected]. Pioneers of Modern Geography Translations Pertaining to German Geographers of the Late Nineteenth and Early Twentieth Centuries Translated and Edited by Robert C. West GEOSCIENCE AND MAN-VOLUME 28-1990 LOUISIANA STATE UNIVERSITY s 62 P5213 iiiiiiiii 10438105 DATE DUE GEOSCIENCE AND MAN Volume 28 PIONEERS OF MODERN GEOGRAPHY Digitized by the Internet Archive in 2017 https://archive.org/details/pioneersofmodern28west GEOSCIENCE & MAN SYMPOSIA, MONOGRAPHS, AND COLLECTIONS OF PAPERS IN GEOGRAPHY, ANTHROPOLOGY AND GEOLOGY PUBLISHED BY GEOSCIENCE PUBLICATIONS DEPARTMENT OF GEOGRAPHY AND ANTHROPOLOGY LOUISIANA STATE UNIVERSITY VOLUME 28 PIONEERS OF MODERN GEOGRAPHY TRANSLATIONS PERTAINING TO GERMAN GEOGRAPHERS OF THE LATE NINETEENTH AND EARLY TWENTIETH CENTURIES Translated and Edited by Robert C. West BATON ROUGE 1990 Property of the LfhraTy Wilfrid Laurier University The Geoscience and Man series is published and distributed by Geoscience Publications, Department of Geography & Anthropology, Louisiana State University.