Leaf Extract
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

Antimicrobial Activity of Three Eryngium L. Species (Apiaceae)
Antimicrobial activity of three Eryngium L. species (Apiaceae) BArBArA Thiem1, OLgA gOśLińskA2, mAłgOrzata kikOwskA1, JArOmir BudziAnOwski1 1department of Pharmaceutical Botany and Plant Biotechnology karol marcinkowski university of medical sciences św. marii magdaleny 14 61-861 Poznań, Poland 2department of Pharmaceutical Bacteriology karol marcinkowski university of medical sciences święcickiego 4 60-781 Poznan, Poland *corresponding author: phone: +4861 6687851, fax: +4861 6687861, e-mail: [email protected] summary The antimicrobial activity of ethanolic extracts from leaves and roots of three Eryngium L. genera (E. planum, E. campestre, E. maritimum) native to Poland was tested by the method of series dilutions against different gram-positive bacteria (two strains) and fungi (five spe- cies). The extracts were analyzed by TLC method to confirm phenolic acids, triterpenoid saponins, flavonoids and acetylenes presence. The antimicrobial activity of extracts com- pared with the reference substance were expressed by minimal inhibitory Concentration (miC). The results have shown that the ethanolic extracts inhibit the growth of Staphylococ- cus aureus and all tested fungi. Key words: Eryngium planum, E. campestre, E. maritimum, antifungal activity, antibacterial activ- ity, leaves and root extracts INTRODUCTION nowadays, many microorganisms have become resistant to commonly used antibiotics and fungicidal agents. resistance to these medicines has led to rese- arch of new sources of bioactive substances against bacteria and fungi. medicinal Antimicrobial activity of three Eryngium L. species (Apiaceae) 53 plants may offer a natural source of antimicrobial bioactive compounds, alternati- ve to antibiotics and fungicidal agents. The genus Eryngium L., belonging to the subfamily Saniculoideae of the family Apiaceae is represented by 317 taxa, widespread in Central Asia, America, Cen- tral and southeast europe. -

Observaciones Sobre La Coleopterofauna Del Cardo Corredor Eryngium Campestre L. (Apiaceae)
Revista gaditana de Entomología, volumen X núm. 1 (2019):117-126 ISSN 2172-2595 Observaciones sobre la coleopterofauna del cardo corredor Eryngium campestre L. (Apiaceae) Rafael Yus Ramos1, Antonio Verdugo Páez2 y Pedro Coello García3 (1) Urbanización “El Jardín nº 22, 29700 Vélez-Málaga (Málaga, España) [email protected] (2) Héroes del Baleares, 10, 3º B. 11100 San Fernando (Cádiz, España) [email protected] (3) Milongas nº 7 (Camposoto) 11100 San Fernando (Cádiz, España) Resumen Se realiza un estudio de la fauna de coleópteros de los órganos aéreos de la apiácea Eryngium campestre L. en la provincia de Cádiz. La coexistencia de diversas especies planteaba un probable problema de competencia interespecífica, por lo que se realizaron observaciones sobre el régimen trófico de cada especie y los órganos vegetales preferentes en donde desarrollan parte de su ciclo biológico. Las observaciones permitieron averiguar determinados comportamientos tróficos mal conocidos hasta la fecha y detalles sobre sus ciclos biológicos, evidenciando los mecanismos usados para evitar la competencia interespecífica en el mismo hábitat. Palabras clave: Coleopterofauna; ciclo biológico; interacciones ecológicas Observations on coleopterofaune of Field Eryngo Eryngium campestre L. (Apiaceae) Abstract A study of the beetle fauna of the air organs of the apiaceous Eryngium campestre L. in the province of Cadiz is carried out. The coexistence of various species posed a probable problem of interspecific competition, so observations were made on the trophic regime of each species and the preferred plant organs where they develop part of their life cycle. The observations made it possible to find out certain badly known trophic behaviors to date and details about their biological cycles, showing the mechanisms used to avoid interspecific competition in the same habitat. -

BSBI Code of Conduct
Code of Conduct for the conservation and enjoyment of wild plants Most people reading this code will support the voluntary plant conservation organisations in their efforts to halt the decline in the native flora of Britain and Ireland and to ensure that all our wild flowering plants, ferns, mosses, liverworts, lichens, algae and fungi remain for future generations to enjoy. Wild plants are a key to the enjoyment of the countryside, primarily for their appeal in their natural surroundings but also because of the pleasure they give photographers, naturalists, flower arrangers and cooks. Generally, uprooting is harmful, but picking with care and in moderation usually does little damage and can foster the appreciation of wild plants, which in turn benefits their conservation. However, in some cases picking can be harmful and it may even be illegal. This leaflet has been written for botanists, teachers and people who wish simply to enjoy wild plants. It aims to indicate where collecting and picking are acceptable and which wild plants should not be taken. Wild plants and the law plants growing in these sites or remove plant material, unless they have first consulted the All wild plants are given some protection statutory conservation agencies (English under the laws of the United Kingdom and Nature, the Countryside Council for Wales, the Republic of Ireland. This leaflet Scottish Natural Heritage or the Environment summarises the relevant legislation in the and Heritage Service, Northern Ireland). It is UK, but does not attempt to cover that of the illegal to pick, uproot or remove plants if by- Republic of Ireland (although a list of species laws are in operation which forbid these protected in Ireland is included). -

G. Domina, P. Marino & G. Castellano
G. Domina, P. Marino & G. Castellano The genus Orobanche (Orobanchaceae) in Sicily Abstract Domina, G., Marino, P. & Castellano, G.: The genus Orobanche (Orobanchaceae) in Sicily. — Fl. Medit. 21: 205-242. 2011. — ISSN: 1120-4052 printed, 2240-4538 online. The taxa of Orobanche occurring in Sicily and on the surrounding islets have been surveyed in the field and in herbaria. In total, 23 species occur in the region. O. litorea is found to be dis- tinct from O. minor and O. thapsoides from O. canescens. O. crenata and O. ramosa are seri- ous pests that cause heavy losses to broad bean and tomato cultures, respectively. Key words: Flora, taxonomy, broomrapes, parasitic plants, Italy. Introduction Most of the European Floras (Webb 1972; Pignatti 1982; Foley 2001, etc.) in their treatment of Orobanche stress its taxonomic difficulty, said to be due to the lack of vegetative organs, to the intra- and inter-population variation, to the short flowering period and to the fragility and loss of colour of herbarium specimens. In reality the worst limitation is the fragility of herbarium specimens. In fact Camarda (1983) in the flowering scape alone observed 42 different characters. Variability must be assessed by studying a large number of individuals from different areas, so as to avoid distin- guishing single individuals or populations as different taxa. The change of colour in dried specimens can be interpreted easily by the practiced observer. Broomrapes are also interesting from an agricultural point of view, when one con- siders that at least 5 species are parasites of important crop species. From 2000 to 2006, the EU funded a research project (COST 849) involving more than 70 researchers, aimed to study and control infestation by Orobanche. -

Novel Serial Extraction Method for Antibacterial and Antifungal Evaluations of the Entire Eryngium Campestre L
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/274376306 Novel serial extraction method for antibacterial and antifungal evaluations of the entire Eryngium campestre L. plant from Jerusalem/ Palestine ARTICLE · JANUARY 2015 READS 20 1 AUTHOR: Nidal Amin Jaradat An-Najah National University 103 PUBLICATIONS 140 CITATIONS SEE PROFILE All in-text references underlined in blue are linked to publications on ResearchGate, Available from: Nidal Amin Jaradat letting you access and read them immediately. Retrieved on: 07 February 2016 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2015, 7(3):905-913 ISSN : 0975-7384 Research Article CODEN(USA) : JCPRC5 Novel serial extraction method for antibacterial and antifungal evaluations of the entire Eryngium campestre L. plant from Jerusalem/ Palestine Nidal Amin Jaradat *1 , Mahdi Mohammad Al khawaja 2, Mahmoud M. Abu-hadid 3 1Pharmaceutical Chemistry and Technology Division, Department of Pharmacy, Faculty of Medicine and Health Sciences, An-Najah National University, Nablus, Palestine 2M. D. Thabet Thabet Governmental Hospital, Tulkarem, Palestine 3Department of Microbiology and Immunology, Faculty of Medicine, Al-Quds University, Jerusalem, Palestine _____________________________________________________________________________________________ ABSTRACT It is noteworthy that the medications isolated directly or indirectly from phytogenic products until the recent time play a primary part in the discovery of drugs. The huge utilizations of antimicrobial agents in medicine have caused directly the development of antibiotics resistant pathogens in various infectious diseases areas, urging the detection for new and effective antimicrobial drugs. This study will be the first of its kind which is designed to evaluate antibacterial and antifungal activities and to estimate exhaustive extraction yields of the aqueous and organic extracts of Eryngium campestre L. -

An Account of Common Broomrape Orobanche Minor (Orobanchaceae) in the British Isles
British & Irish Botany 2(3): 223-239, 2020 An account of Common Broomrape Orobanche minor (Orobanchaceae) in the British Isles C.J. Thorogood1,2*, Fred Rumsey3 1University of Oxford Botanic Garden, Oxford, UK; 2Department of Plant Sciences, University of Oxford, Oxford, UK; 3Natural History Museum, London, UK. *Corresponding author: Chris Thorogood: [email protected] This pdf constitutes the Version of Record published on 31st August 2020 Abstract Common broomrape (Orobanche minor Sm.) is the most widespread and variable species in the British Isles and is the subject of much taxonomic confusion. Poor preservation in herbaria, coupled with the presence of cryptic host-specific races, have contributed to this. Here we review the taxonomic status of infraspecific taxa of O. minor in the British Isles, and provide a revised identification key, informed by morphology, ecology and molecular data. We describe two new varieties of O. minor s.l. that are ecologically distinct and discuss within the broader context of cryptic taxa in the subsection Minores (Beck-Mannagetta) Teryokhin in the British Isles and continental Europe. We suggest that delineating taxa objectively and reliably will be important for informing conservation priorities. Host identity and ecology, besides morphology, are essential considerations when identifying infraspecific taxa in this taxonomically challenging species. Keywords: Taxonomy; host race; parasitic plant Introduction Broomrapes (genus Orobanche L.) are renowned for being taxonomically challenging. Many of the characters useful for identification, for example stigma and corolla colour, are lost upon drying. Coupled with inadequate field notes, herbarium specimens are often determined incorrectly (Rumsey & Jury, 1991). Of the 14 species that occur in the British Isles, common broomrape (Orobanche minor Sm.) is the most widespread and variable (Figs. -
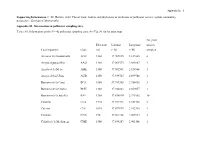
Supporting Information. C. M. Herrera. 2020. Flower Traits, Habitat, and Phylogeny As Predictors of Pollinator Service: a Plant Community Perspective
Appendix S1 – 1 Supporting Information. C. M. Herrera. 2020. Flower traits, habitat, and phylogeny as predictors of pollinator service: a plant community perspective. Ecological Monographs. Appendix S1. Information on pollinator sampling sites. TABLE S1. Information on the N = 42 pollinator sampling sites. See Fig. S1 for location map. No. plant Elevation Latitude Longitude species Local toponym Code (m) (º N) (º W) sampled Arenales del Guadalentín AGU 1360 37.909835 2.837405 4 Arroyo Aguaderillos AAG 1180 37.961573 2.883887 1 Arroyo de la Mesa AME 1100 37.902591 2.928966 1 Arroyo de los Ubios AUB 1250 37.939725 2.899766 1 Barranco de la Canal BCA 1580 37.789385 2.956438 3 Barranco de la Charca BCH 1360 37.942843 2.859057 1 Barranco de la Juanfría BJU 1380 37.836814 2.975302 10 Calarilla CLA 1710 37.944316 2.851708 2 Calerón CLE 1075 37.897973 2.942710 1 Cantalar CAN 770 37.964158 2.907974 3 Cañada de la Medianega CME 1580 37.894287 2.903146 1 Appendix S1 – 2 Cañada Pajarera CPA 1640 37.927456 2.805615 1 Collado del Calvario CCA 1420 37.950202 2.885484 1 Collado Zamora CZA 1420 37.839329 2.999615 2 Coto del Valle CVA 850 37.930542 2.928517 7 Cuesta del Bazar CBA 1330 37.906523 2.907470 1 Cuevas Bermejas CBE 1160 37.966959 2.851117 5 Fuente Bermejo FBE 1550 37.932268 2.840672 14 Fuente la Reina FRE 1460 37.941698 2.833632 2 Guadalentín GUA 1310 37.900404 2.836944 8 Hoyos de Muñoz HMU 1110 37.984831 2.876597 3 La Cabrilla LCA 1600 37.932364 2.780637 5 La Fresnedilla LFR 1080 37.980387 2.855875 2 La Mesa LME 1610 37.889823 2.913788 2 Las -

Sympetaly in Apiales (Apiaceae, Araliaceae, Pittosporaceae)
South African Journal of Botany 2004, 70(3): 458–467 Copyright © NISC Pty Ltd Printed in South Africa — All rights reserved SOUTH AFRICAN JOURNAL OF BOTANY ISSN 0254–6299 Sympetaly in Apiales (Apiaceae, Araliaceae, Pittosporaceae) C Erbar* and P Leins Heidelberg Institute of Plant Sciences (HIP) — Biodiversity and Plant Systematics, University of Heidelberg, Im Neuenheimer Feld 345, D-69120 Heidelberg, Germany * Corresponding author, e-mail: [email protected] Received 10 March 2003, accepted in revised form 24 October 2003 In all recent molecular sequence based analyses Pittosporum) the corollas are initiated from a continu- Apiales come out to be placed within a broadly defined ous ring primordium corresponding exactly to the group Asteridae. Within ‘euasterids II’ Apiales development in Campanulales–Asterales and (Apiaceae, Araliaceae, Pittosporaceae, Aralidiaceae, as Dipsacales. Only in Pittosporaceae further growth of well as some former cornaceous taxa) form a mono- this primordium results in a weak sympetaly in adult phyletic group in a position close to Asterales– flowers. Molecular data suggest that the subfamily Campanulales and Dipsacales. Also from a floral devel- Hydrocotyloideae is polyphyletic, with Hydrocotyle opmental point of view the mostly choripetalous Apiales belonging to the lineage not placed within Apiaceae but are not out of place among these sympetalous orders: more closely related to Araliaceae, a position fitting well In members of Apiales (Apiaceae: Hydrocotyle; with the mode of formation of the corolla. Araliaceae: Aralia, Hedera; Pittosporaceae: Sollya, Introduction Flowers with a corolla tube can be found in many members tube formation, a corolla tube ontogenetically can be initiat- of the angiosperms, but are concentrated in the upper evo- ed extremely early, namely before the petal primordia arise. -

TAXON:Eryngium Foetidum L. SCORE:15.0 RATING:High Risk
TAXON: Eryngium foetidum L. SCORE: 15.0 RATING: High Risk Taxon: Eryngium foetidum L. Family: Apiaceae Common Name(s): culantro Synonym(s): Eryngium antihystericum Rottler false coriander fitweed long coriander Mexican coriander sawtooth coriander shadow beni spiny coriander spiritweed stinkweed Assessor: No Assessor Status: Assessor Approved End Date: 30 Apr 2018 WRA Score: 15.0 Designation: H(HPWRA) Rating: High Risk Keywords: Biennial Herb, Spiny, Edible, Disturbance Weed, Shade-Tolerant Qsn # Question Answer Option Answer 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? 103 Does the species have weedy races? Species suited to tropical or subtropical climate(s) - If 201 island is primarily wet habitat, then substitute "wet (0-low; 1-intermediate; 2-high) (See Appendix 2) High tropical" for "tropical or subtropical" 202 Quality of climate match data (0-low; 1-intermediate; 2-high) (See Appendix 2) High 203 Broad climate suitability (environmental versatility) y=1, n=0 y Native or naturalized in regions with tropical or 204 y=1, n=0 y subtropical climates Does the species have a history of repeated introductions 205 y=-2, ?=-1, n=0 y outside its natural range? 301 Naturalized beyond native range y = 1*multiplier (see Appendix 2), n= question 205 y 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see Appendix 2) y 303 Agricultural/forestry/horticultural weed 304 Environmental weed n=0, y = 2*multiplier (see Appendix 2) n 305 Congeneric weed n=0, y = 1*multiplier (see Appendix 2) y 401 Produces spines, thorns or burrs y=1, n=0 y 402 Allelopathic Creation Date: 30 Apr 2018 (Eryngium foetidum L.) Page 1 of 19 TAXON: Eryngium foetidum L. -

One Pot Green Synthesis and Characterization of Trimetallic Oxide Cucrnio Nps Using Eryngium Campestre Leaf Extract
International Proceedings of Chemical, Biological and Environmental Engineering, V0l. 101 (2017) DOI: 10.7763/IPCBEE. 2017. V101. 9 One Pot Green Synthesis and Characterization of Trimetallic Oxide CuCrNiO NPs Using Eryngium Campestre Leaf Extract Zahra Vaseghi 1, Omid Tavakoli 1 and Ali Nematollahzadeh 2 1 School of Chemical Engineering, College of Engineering, University of Tehran, Iran 2 Chemical Engineering Department, University of Mohaghegh Ardabili, P. O. Box, 179, Ardabil, Iran Abstract. Trimetallic oxide CuCrNiO nanoparticles (NPs) were successfully synthesized from their precursor salts including CuSO4.5H2O, Cr(NO3)3.9H2O, and Ni(NO3)2.6H2O in a facile and green method using aqueous leaf extract of Eryngium campestre as reducing and stabilizing agents. NPs were characterized using UV-vis spectroscopy, FTIR, EDX, XRD, and FESEM showing that trimetallic oxide NPs were synthesized in the form of nanoplates with the thickness of 18.73 nm and crystallite size of 17.39 nm. Keywords: green synthesis, trimetallic NPs, aqueous leaf extract, characterization 1. Introduction In recent years, production of NPs through green route has received considerable attention from researchers all over the world. Choice of solvent, reducing, and stabilizing/capping agent are three main criteria which should be taken into consideration in the green synthesis of NPs [1]-[3]. Typically, in green synthesis approach the reducing agents can be applied from two main categories of microbial (microorganism mediated) and phyto (plant mediated) [1], [4]. Plant mediated synthesis of NPs has some advantages including increased synthesis rate, enhanced monodispersity and stability of NPs, and decreased requirement for complex treatment in comparison to microbial synthesis using bacteria, fungi, yeast, and algae [1]. -

Population Genetic Structure and Plant Fitness of Natural and Ex Situ
Population genetic structure and plant fitness of natural and ex situ populations in Silene chlorantha (W ILLD .) EHRH . and Silene otites (L.) WIBEL Dissertation zur Erlangung des akademischen Grades des Doktors der Naturwissenschaften (Dr. rer. nat.) eingereicht im Fachbereich Biologie, Chemie, Pharmazie der Freien Universität Berlin vorgelegt von Daniel Lauterbach aus Brandenburg an der Havel 2012 Die Arbeit wurde im Zeitraum von Juni 2008 bis Februar 2012 an der Zentraleinrichtung Botanischer Garten und Botanisches Museum Berlin - Dahlem, Freie Universität Berlin unter der Leitung von Herrn Prof. Dr. Thomas Borsch angefertigt. 1. Gutachter: Prof. Dr. Thomas Borsch 2. Gutachter: Prof. Dr. Ingo Kowarik Disputation am 04.07.2012 Index Table of contents 1 General Introduction .................................................................................................... 1 1.1 Effects of land-use changes on dry grasslands .................................................. 1 1.2 The study species: Silene chlorantha (WILLD .) EHRH . and Silene otites (L.) WIBEL 2 1.3 Population genetic structure and plant fitness ................................................... 7 1.4 Ex situ plant conservation .................................................................................. 10 1.5 Comments to the structure of the presented thesis .......................................... 12 2 Genetic population structure, fitness variation and the importance of population history in remnant populations of the endangered plant Silene chlorantha