Comparative Genomics of the Major Parasitic Worms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular Characterization of Β-Tubulin Isotype-1 Gene of Bunostomum Trigonocephalum
Int.J.Curr.Microbiol.App.Sci (2018) 7(7): 3351-3358 International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 7 Number 07 (2018) Journal homepage: http://www.ijcmas.com Original Research Article https://doi.org/10.20546/ijcmas.2018.707.390 Molecular Characterization of β-Tubulin Isotype-1 Gene of Bunostomum trigonocephalum Ravi Kumar Khare1, A. Dixit3, G. Das4, A. Kumar1, K. Rinesh3, D.S. Khare4, D. Bhinsara1, Mohar Singh2, B.C. Parthasarathi2, P. Dipali2, M. Shakya5, J. Jayraw5, D. Chandra2 and M. Sankar1* 1Division of Temperate Animal Husbandry, ICAR- IVRI, Mukteswar, India 2IVRI, Izatnagar, India 3College of Veterinary Science and A.H., Rewa, India 4College of Veterinary Sciences and A.H., Jabalpur, India 5College of Veterinary Sciences and A.H., Mhow, India *Corresponding author ABSTRACT The mechanism of benzimidazoles resistance is linked to single nucleotide polymorphisms (SNPs) on beta -tubulin isotype-1 gene. The three known SNPs responsible for BZ K e yw or ds resistance are F200Y, F167Y and E198A on the beta-tubulin isotype-1. The present study was aimed to characterize beta-tubulin isotype-1 gene of Bunostomum trigonocephalum, Benzimidazole for identifying variations on possible mutation sites. The adult parasites were collected resistance, Beta from Mukteswar, Uttarakhand. The parasites were thoroughly examined morphologically tubulin, and male parasites were subjected for RNA isolation. Complementary DNA (cDNA) was Bunostomum synthesised from total RNA using OdT. The PCR was performed using cDNA and self trigonocephalum, Small ruminants designed degenerative primers. The purified PCR amplicons were cloned into pGEMT easy vector and custom sequenced. The obtained sequences were analysed using DNA Article Info STAR, MEGA7.0 and Gene tool software. -

Gastrointestinal Parasites of Maned Wolf
http://dx.doi.org/10.1590/1519-6984.20013 Original Article Gastrointestinal parasites of maned wolf (Chrysocyon brachyurus, Illiger 1815) in a suburban area in southeastern Brazil Massara, RL.a*, Paschoal, AMO.a and Chiarello, AG.b aPrograma de Pós-Graduação em Ecologia, Conservação e Manejo de Vida Silvestre – ECMVS, Universidade Federal de Minas Gerais – UFMG, Avenida Antônio Carlos, 6627, CEP 31270-901, Belo Horizonte, MG, Brazil bDepartamento de Biologia da Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Universidade de São Paulo – USP, Avenida Bandeirantes, 3900, CEP 14040-901, Ribeirão Preto, SP, Brazil *e-mail: [email protected] Received: November 7, 2013 – Accepted: January 21, 2014 – Distributed: August 31, 2015 (With 3 figures) Abstract We examined 42 maned wolf scats in an unprotected and disturbed area of Cerrado in southeastern Brazil. We identified six helminth endoparasite taxa, being Phylum Acantocephala and Family Trichuridae the most prevalent. The high prevalence of the Family Ancylostomatidae indicates a possible transmission via domestic dogs, which are abundant in the study area. Nevertheless, our results indicate that the endoparasite species found are not different from those observed in protected or least disturbed areas, suggesting a high resilience of maned wolf and their parasites to human impacts, or a common scenario of disease transmission from domestic dogs to wild canid whether in protected or unprotected areas of southeastern Brazil. Keywords: Chrysocyon brachyurus, impacted area, parasites, scat analysis. Parasitas gastrointestinais de lobo-guará (Chrysocyon brachyurus, Illiger 1815) em uma área suburbana no sudeste do Brasil Resumo Foram examinadas 42 fezes de lobo-guará em uma área desprotegida e perturbada do Cerrado no sudeste do Brasil. -

Managing Hookworms in the Landscape 1
Archival copy: for current recommendations see http://edis.ifas.ufl.edu or your local extension office. ENY-017 Managing Hookworms in the Landscape 1 Robert A. Dunn and Ellis C. Greiner2 "Hookworms" properly refers to many genera of • A. tubaeforme is the common hookworm of nematodes in the Family Ancylostomatidae of the cats, distributed world-wide; similar to A. Order Strongylida, but this discussion addresses caninum, but generally smaller. primarily those in the genus Ancylostoma, which many animal health professionals consider to be the • Uncinaria stenocephala occurs in the small most important genus of hookworms. This genus intestine of dogs, cats, foxes, wolves, and related includes the most common hookworms of domestic carnivores. It is occasionally recovered from dogs and cats in tropical and warm temperate stray dogs in Florida, but may not occur climates, the hookworms with which most people in endemically here -- the infections that are Florida come into contact. The "northern carnivore detected may have occurred farther north, before hookworm," Uncinaria stenocephala, also occurs in the host animals came to Florida. Florida but much less frequently than Ancylostoma Importance as Animal and Human spp. Parasites Four species are significant in Florida; the three Ancylostoma spp. represent 90 - 95% of hookworms Widely distributed wherever dogs and cats are identified here: kept as pets, hookworms are found commonly in the small intestines of hosts in which they can complete • Ancylostoma caninum, the dog hookworm, is their life cycles. Hookworms suck blood from the found in the small intestine of dogs, foxes, intestinal wall. The degree of blood sucking varies coyotes, wolves, bears, and other wild carnivores among these hookworms. -

Semi-Domesticated Dogs As a Potential Reservoir for Zoonotic Hookworms in Bangkok, Thailand
Veterinary World, EISSN: 2231-0916 RESEARCH ARTICLE Available at www.veterinaryworld.org/Vol.13/May-2020/12.pdf Open Access Semi-domesticated dogs as a potential reservoir for zoonotic hookworms in Bangkok, Thailand Jutamas Wongwigkan1,2,3 and Tawin Inpankaew1,2,3 1. Center for Agricultural Biotechnology, Kasetsart University, Kamphaeng Saen Campus, Nakhon Pathom, Thailand; 2. Center of Excellence on Agricultural Biotechnology: (AG-BIO/PERDO-CHE), Bangkok, Thailand; 3. Department of Parasitology, Faculty of Veterinary Medicine, Kasetsart University, Bangkok, Thailand. Corresponding author: Tawin Inpankaew, e-mail: [email protected] Co-author: JW: [email protected] Received: 12-12-2019, Accepted: 13-04-2020, Published online: 16-05-2020 doi: www.doi.org/10.14202/vetworld.2020.909-915 How to cite this article: Wongwigkan J, Inpankaew T (2020) Semi-domesticated dogs as a potential reservoir for zoonotic hookworms in Bangkok, Thailand, Veterinary World, 13(5): 909-915. Abstract Background and Aim: Hookworms are parasitic nematodes that live in the small intestine of their mammalian hosts including humans, dogs, and cats. This study was conducted to determine the prevalence and perform genetic characterization of hookworms using molecular techniques and to elucidate the risk factors associated with hookworm infections among semi-domesticated dogs residing in temples in the Bangkok Metropolitan Area, Thailand. Materials and Methods: A total of 500 fecal samples were collected from semi-domesticated dogs from 91 temples in 48 districts of Bangkok. DNA was extracted and screened using internal transcribed spacer polymerase chain reaction-restriction fragment length polymorphism. In addition, samples positive for Ancylostoma ceylanicum were further characterized at the haplotype level based on the analysis of the mitochondrial cytochrome oxidase-1 gene (cox1). -

Testing the Hypothesis of Recent Population Expansions in Nematode Parasites of Human-Associated Hosts
Heredity (2005) 94, 426–434 & 2005 Nature Publishing Group All rights reserved 0018-067X/05 $30.00 www.nature.com/hdy Testing the hypothesis of recent population expansions in nematode parasites of human-associated hosts DA Morrison and J Ho¨glund Department of Parasitology (SWEPAR), National Veterinary Institute and Swedish University of Agricultural Sciences, 751 89 Uppsala, Sweden It has been predicted that parasites of human-associated prediction. However, it is likely that the situation is more organisms (eg humans, domestic pets, farm animals, complicated than the simple hypothesis test suggests, and agricultural and silvicultural plants) are more likely to show those species that do not fit the predicted general pattern rapid recent population expansions than are parasites of provide interesting insights into other evolutionary processes other hosts. Here, we directly test the generality of this that influence the historical population genetics of host– demographic prediction for species of parasitic nematodes parasite relationships. These processes include the effects of that currently have mitochondrial sequence data available in postglacial migrations, evolutionary relationships and possi- the literature or the public-access genetic databases. Of the bly life-history characteristics. Furthermore, the analysis 23 host/parasite combinations analysed, there are seven highlights the limitations of this form of bioinformatic data- human-associated parasite species with expanding popula- mining, in comparison to controlled experimental -

Ancylostomiasis (Hookworm Disease)
EAZWV Transmissible Disease Fact Sheet Sheet No. 104 ANCYLOSTOMIASIS (HOOKWORM DISEASE) ANIMAL TRANS- CLINICAL FATAL TREATMENT PREVENTION GROUP MISSION SIGNS DISEASE ? & CONTROL AFFECTED Percutaneous- rarely In houses Pongidae, ly Larva migrans Mebendazol Cercopitheci (in man also symptoms, dae, perorally via dyspnea, in zoos Cebidae. breast milk). diarrhea. Fact sheet compiled by Last update Manfred Brack, formerly German Primate Center, 22.11..2008 Göttingen/Germany. Susceptible animal groups Gorilla gorilla,Pan troglodytes, Hylobates sp.,Papio sp.,Macaca mulatta,Cercopithecus mona : A.duodenale ; Cebus capucinus, Ateles sp.,Erythrocebus patas, Cercopithecus mona : Necator americanus. Causative organism Ancylostoma duodenale, Necator americanus (Nematoda, Strongylina: Ancylostomatidae). Zoonotic potential Yes. Distribution A.duodenale : world-wide, predominantly in tropical/subtropical S.E.Asia and America; N.americanus : tropical and subtropical rain forests. Transmission Percutaneously by filariform ( 3 rd stage) larvae. Incubation period Clinical symptoms Pot belly syndrome, apnea, cutaneous larva migrans, persistent diarrhea, in man also anemia. Post mortem findings Not reported in nonhuman primates. Diagnosis Ovodiagnosis ( cave: ancylostomatid eggs may be confused with the very similar oesophagostomid eggs!), followed by fecoculture of filariform larvae (Harada-Mori technique). Material required for laboratory analysis Relevant diagnostic laboratories Treatment Mebendazole (2 x 15 mg / kg or 10 x 3 mg / kg). Ivermectin is almost -

Redalyc.First Record of Intestinal Parasites in a Wild Population Of
Revista Brasileira de Parasitologia Veterinária ISSN: 0103-846X [email protected] Colégio Brasileiro de Parasitologia Veterinária Brasil Srbek-Araujo, Ana Carolina; Costa Santos, Juliana Lúcia; Medeiros de Almeida, Viviane; Pezzi Guimarães, Marcos; Garcia Chiarello, Adriano First record of intestinal parasites in a wild population of jaguar in the Brazilian Atlantic Forest Revista Brasileira de Parasitologia Veterinária, vol. 23, núm. 3, julio-septiembre, 2014, pp. 393-398 Colégio Brasileiro de Parasitologia Veterinária Jaboticabal, Brasil Available in: http://www.redalyc.org/articulo.oa?id=397841493016 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Research note Braz. J. Vet. Parasitol., Jaboticabal, v. 23, n. 3, p. 393-398, jul.-set. 2014 ISSN 0103-846X (Print) / ISSN 1984-2961 (Electronic) Doi: http://dx.doi.org/10.1590/S1984-29612014065 First record of intestinal parasites in a wild population of jaguar in the Brazilian Atlantic Forest Primeiros registros de parasitos intestinais em uma população silvestre de onça-pintada na Mata Atlântica Brasileira Ana Carolina Srbek-Araujo1,2*; Juliana Lúcia Costa Santos3; Viviane Medeiros de Almeida3; Marcos Pezzi Guimarães3; Adriano Garcia Chiarello4 1Programa de Pós-graduação em Ecologia de Ecossistemas, Universidade Vila Velha – UVV, Vila Velha, ES, -
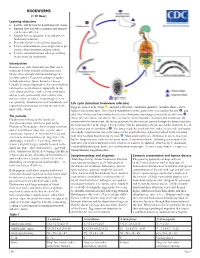
HOOKWORMS (1 CE Hour) Learning Objectives !! List the Risk Factors for Hookworm Infections
HOOKWORMS (1 CE Hour) Learning objectives ! List the risk factors for hookworm infections. ! Explain how and where humans and animals can become infected. ! Explain how to diagnose, treat and prevent hookworm infection. ! Describe the life cycle of these parasites. ! List recommendations you can provide to pet owners about treatment and prevention. ! List the contraindications when prescribing medications for hookworm. Introduction Zoonoses are infectious diseases that can be transmitted from animals to humans, most likely when animals and human beings are in close contact. Causative groups of agents include parasites, fungi, bacteria, viruses. Usually of minor importance, they nevertheless can lead to severe disease, especially in the very young and those with certain conditions, and to death, particularly with certain viral diseases (such as rabies, hemorrhagic fevers, encephalitis). Hookworms exist worldwide and Life cycle (Intestinal hookworm infection) cross from carnivorous animals to man in all Eggs are passed in the stool , and under favorable conditions (moisture, warmth, shade), larvae parts of the world. hatch in one to two days. The released rhabditiform larvae grow in the feces and/or the soil , and The parasite after 5 to 10 days (and two molts) they become filariform (third-stage) larvae that are infective . These infective larvae can survive three to four weeks in favorable environmental conditions. On Hookworms belong to the family of contact with the human host, the larvae penetrate the skin and are carried through the blood vessels to Ancylostomatidae which are part of the the heart and then to the lungs. They penetrate into the pulmonary alveoli, ascend the bronchial tree to Phylum of Nematodes: Ancylostoma caninum the pharynx and are swallowed . -

The Mitochondrial Genome of Angiostrongylus Mackerrasae As a Basis for Molecular, Epidemiological and Population Genetic Studies Mahdis Aghazadeh1,2* , Rebecca J
Aghazadeh et al. Parasites & Vectors (2015) 8:473 DOI 10.1186/s13071-015-1082-0 RESEARCH Open Access The mitochondrial genome of Angiostrongylus mackerrasae as a basis for molecular, epidemiological and population genetic studies Mahdis Aghazadeh1,2* , Rebecca J. Traub3, Namitha Mohandas3, Kieran V. Aland4, Simon A. Reid5, James S. McCarthy2,5 and Malcolm K. Jones1,2 Abstract Background: Angiostrongylus mackerrasae is a metastrongyloid nematode endemic to Australia, where it infects the native bush rat, Rattus fuscipes. This lungworm has an identical life cycle to that of Angiostrongylus cantonensis, a leading cause of eosinophilic meningitis in humans. The ability of A. mackerrasae to infect non-rodent hosts, specifically the black flying fox, raises concerns as to its zoonotic potential. To date, data on the taxonomy, epidemiology and population genetics of A. mackerrasae are unknown. Here, we describe the mitochondrial (mt) genome of A. mackerrasae with the aim of starting to address these knowledge gaps. Methods: The complete mitochondrial (mt) genome of A. mackerrasae was amplified from a single morphologically identified adult worm, by long-PCR in two overlapping amplicons (8 kb and 10 kb). The amplicons were sequenced using the MiSeq Illumina platform and annotated using an in-house pipeline. Amino acid sequences inferred from individual protein coding genes of the mt genomes were concatenated and then subjected to phylogenetic analysis using Bayesian inference. Results: The mt genome of A. mackerrasae is 13,640 bp in size and contains 12 protein coding genes (cox1-3,nad1-6, nad4L,atp6andcob), and two ribosomal RNA (rRNA) and 22 transfer RNA (tRNA) genes. -

Molecular Epidemiology of Hookworm Infection in the Ashanti Region, Ghana
Yale University EliScholar – A Digital Platform for Scholarly Publishing at Yale Public Health Theses School of Public Health January 2020 Molecular Epidemiology Of Hookworm Infection In The Ashanti Region, Ghana. Emma Allen [email protected] Follow this and additional works at: https://elischolar.library.yale.edu/ysphtdl Recommended Citation Allen, Emma, "Molecular Epidemiology Of Hookworm Infection In The Ashanti Region, Ghana." (2020). Public Health Theses. 1916. https://elischolar.library.yale.edu/ysphtdl/1916 This Open Access Thesis is brought to you for free and open access by the School of Public Health at EliScholar – A Digital Platform for Scholarly Publishing at Yale. It has been accepted for inclusion in Public Health Theses by an authorized administrator of EliScholar – A Digital Platform for Scholarly Publishing at Yale. For more information, please contact [email protected]. Molecular epidemiology of hookworm infection in the Ashanti Region, Ghana. Emma Allen 2020 2020 Master of Public Health Epidemiology of Microbial Diseases Michael Cappello, MD Debbie Humphries, PhD, MPH I. Abstract INTRODUCTION: Parasitic helminth infections have persisted in resource-limited settings around the world, leading to greater numbers of people experiencing sequelae including malnutrition, anemia, and impaired growth and cognitive function among children. Despite periodic deworming efforts, a high burden of disease remains in sub-Saharan Africa. Establishing baseline prevalence of these infections can inform local and national deworming campaign objectives as well as tailor additional interventions accordingly. Here we describe our efforts to determine baseline prevalence and analyze potential risk factors for infection in Ashanti Region, Ghana. OBJECTIVES: The primary objective of this study was to characterize the molecular epidemiology of hookworm and schistosomiasis co-infections among communities surrounding Lake Bosumtwe in Ashanti Region, Ghana. -

Hookworms Family: Ancylostomatidae Ancylostoma Duodenale
Lect: 5 Nematoda 3rd class Dr.Omaima I. M. Hookworms Family: Ancylostomatidae Ancylostoma duodenale Along with its range of definitive hosts, Ancylostoma duodenale also has a range of paratenic hosts of canids and felids, where it may remain for intervals of time until it reaches the definitive host. In the paratenic host it may survive in the muscles where it is then transferred to humans via undercooked meat, including rabbit, lamb, beef, and pork. The eggs of Ancylostoma duodenale are still within the muscle and are ingested with the meat, allowing for the adults to develop within the intestinal tract. Main properties Ancylostoma duodenale is an S-shaped worm because of its flexure at the frontal end. The worm is pinkish-white. Adult male hookworms range in size from 8-11 mm long, whereas adult females range in size from 10- 13 mm long. This species is dimorphic, with the males having bursa characteristics and needle-like spicules with small tips, which are distally fused. Females have a vulva located approximately one-third of the body length from the posterior end. Both male and female hookworms have two powerful ventral teeth in the adult forms of the parasite, one along each side of the buccal capsule; smaller pairs of teeth are located deeper in the capsule. http://cvet.tu.edu.iq Hookworm eggs have a thin shell and the larvae possess amphids, large paired sensilla on each side of the mouth, which allow them to locate their host. The larvae are rod-shaped and are about 0.004 cm long. -

Mitochondrial DNA Reveals Species Composition and Phylogenetic Relationships of Hookworms in Northeastern Brazil T
Infection, Genetics and Evolution 68 (2019) 105–112 Contents lists available at ScienceDirect Infection, Genetics and Evolution journal homepage: www.elsevier.com/locate/meegid Research paper Mitochondrial DNA reveals species composition and phylogenetic relationships of hookworms in northeastern Brazil T Kerla Joeline Lima Monteiroa,b,1, Lauren Hubert Jaegera,1, Beatriz Coronato Nunesa, Deiviane Aparecida Calegara, Elis Regina Chaves dos Reisa, Polyanna Araújo Alves Bacelara,b, ⁎ Jéssica Pereira dos Santosa,b, Márcio Neves Bóiac, Filipe Anibal Carvalho-Costaa,b, a Laboratory of Epidemiology and Molecular Systematics, Oswaldo Cruz Institute, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil b Oswaldo Cruz Foundation, Teresina, Piauí, Brazil c Laboratory of Biology and Parasitology of Wild Reservoir Mammals, Oswaldo Cruz Institute, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil ARTICLE INFO ABSTRACT Keywords: Hookworm infection persists focally in rural communities in Brazil. In this study, we analyze the mitochondrial Hookworm nucleotide sequences obtained from hookworms infecting humans in order to characterize species composition cox1 and assess their genetic diversity and phylogenetic relationships. Field expeditions and cross-sectional surveys Northeastern were carried out in three Brazilian municipalities from 2013 to 2017: Nossa Senhora de Nazaré (n = 605) and Brazil. Teresina (n = 297), in the state of Piauí, and Russas (n = 213) in the State of Ceará. Parasitological methods were used to evaluate fecal samples. Hookworm-positive samples had a partial mtDNA cox1 amplified and sequenced. Maximum-likelihood and Bayesian analysis demonstrated two strongly-supported clades, including Group A, corresponding to Necator americanus, and Groups B and C, corresponding to Necator sp. Group A was divided into three main clusters: A1 grouped with Asian sequences, A2 grouped with African sequences, and A3 had only Asian sequences.