DIAGEO Plc, of 1933 and SECTION 21C of the SECURITIES EXCHANGE ACT of 1934, Respondent
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Corporate Governance
Strategic report Governance and remuneration Financial statements Investor information Corporate Governance In this section Chairman’s Governance statement 78 The Board 80 Corporate Executive Team 83 Board architecture 85 Board roles and responsibilities 86 Board activity and principal decisions 87 Our purpose, values and culture 90 The Board’s approach to engagement 91 Board performance 94 Board Committee information 96 Our Board Committee reports 97 Section 172 statement 108 Directors’ report 109 GSK Annual Report 2020 77 Chairman’s Governance statement In last year’s Governance statement, I explained that our primary Education and focus on Science objective for 2020 was to ensure there was clarity between the Given the critical importance of strengthening the pipeline, Board and management on GSK’s execution of strategy and its the Board has benefitted from devoting a higher proportion of operational priorities. We have aligned our long-term priorities its time in understanding the science behind our strategy and of Innovation, Performance and Trust powered by culture testing its application. It is important that the Board has a and agreed on the metrics to measure delivery against them. working understanding of the key strategic themes upon The Board’s annual cycle of meetings ensures that all major which our R&D strategy is based. These themes have been components of our strategy are reviewed over the course complemented by Board R&D science thematic deep dives. of the year. Our focus was on the fundamentals of our strategy: human The COVID-19 pandemic impacted and dominated all our genetics, the immune system and AI and ML, as well as to lives for the majority of 2020. -

To Arrive at the Total Scores, Each Company Is Marked out of 10 Across
BRITAIN’S MOST ADMIRED COMPANIES THE RESULTS 17th last year as it continues to do well in the growing LNG business, especially in Australia and Brazil. Veteran chief executive Frank Chapman is due to step down in the new year, and in October a row about overstated reserves hit the share price. Some pundits To arrive at the total scores, each company is reckon BG could become a take over target as a result. The biggest climber in the top 10 this year is marked out of 10 across nine criteria, such as quality Petrofac, up to fifth from 68th last year. The oilfield of management, value as a long-term investment, services group may not be as well known as some, but it is doing great business all the same. Its boss, Syrian- financial soundness and capacity to innovate. Here born Ayman Asfari, is one of the growing band of are the top 10 firms by these individual measures wealthy foreign entrepreneurs who choose to make London their operating base and home, to the benefit of both the Exchequer and the employment figures. In fourth place is Rolls-Royce, one of BMAC’s most Financial value as a long-term community and environmental soundness investment responsibility consistent high performers. Hardly a year goes past that it does not feature in the upper reaches of our table, 1= Rightmove 9.00 1 Diageo 8.61 1 Co-operative Bank 8.00 and it has topped its sector – aero and defence engi- 1= Rotork 9.00 2 Berkeley Group 8.40 2 BASF (UK & Ireland) 7.61 neering – for a decade. -

Ftse4good UK 50
2 FTSE Russell Publications 19 August 2021 FTSE4Good UK 50 Indicative Index Weight Data as at Closing on 30 June 2021 Index weight Index weight Index weight Constituent Country Constituent Country Constituent Country (%) (%) (%) 3i Group 0.81 UNITED GlaxoSmithKline 5.08 UNITED Rentokil Initial 0.67 UNITED KINGDOM KINGDOM KINGDOM Anglo American 2.56 UNITED Halma 0.74 UNITED Rio Tinto 4.68 UNITED KINGDOM KINGDOM KINGDOM Antofagasta 0.36 UNITED HSBC Hldgs 6.17 UNITED Royal Dutch Shell A 4.3 UNITED KINGDOM KINGDOM KINGDOM Associated British Foods 0.56 UNITED InterContinental Hotels Group 0.64 UNITED Royal Dutch Shell B 3.75 UNITED KINGDOM KINGDOM KINGDOM AstraZeneca 8.25 UNITED International Consolidated Airlines 0.47 UNITED Schroders 0.28 UNITED KINGDOM Group KINGDOM KINGDOM Aviva 1.15 UNITED Intertek Group 0.65 UNITED Segro 0.95 UNITED KINGDOM KINGDOM KINGDOM Barclays 2.1 UNITED Legal & General Group 1.1 UNITED Smith & Nephew 0.99 UNITED KINGDOM KINGDOM KINGDOM BHP Group Plc 3.2 UNITED Lloyds Banking Group 2.39 UNITED Smurfit Kappa Group 0.74 UNITED KINGDOM KINGDOM KINGDOM BT Group 1.23 UNITED London Stock Exchange Group 2.09 UNITED Spirax-Sarco Engineering 0.72 UNITED KINGDOM KINGDOM KINGDOM Burberry Group 0.6 UNITED Mondi 0.67 UNITED SSE 1.13 UNITED KINGDOM KINGDOM KINGDOM Coca-Cola HBC AG 0.37 UNITED National Grid 2.37 UNITED Standard Chartered 0.85 UNITED KINGDOM KINGDOM KINGDOM Compass Group 1.96 UNITED Natwest Group 0.77 UNITED Tesco 1.23 UNITED KINGDOM KINGDOM KINGDOM CRH 2.08 UNITED Next 0.72 UNITED Unilever 7.99 UNITED KINGDOM KINGDOM -

Constituents & Weights
2 FTSE Russell Publications 19 August 2021 FTSE 100 Indicative Index Weight Data as at Closing on 30 June 2021 Index weight Index weight Index weight Constituent Country Constituent Country Constituent Country (%) (%) (%) 3i Group 0.59 UNITED GlaxoSmithKline 3.7 UNITED RELX 1.88 UNITED KINGDOM KINGDOM KINGDOM Admiral Group 0.35 UNITED Glencore 1.97 UNITED Rentokil Initial 0.49 UNITED KINGDOM KINGDOM KINGDOM Anglo American 1.86 UNITED Halma 0.54 UNITED Rightmove 0.29 UNITED KINGDOM KINGDOM KINGDOM Antofagasta 0.26 UNITED Hargreaves Lansdown 0.32 UNITED Rio Tinto 3.41 UNITED KINGDOM KINGDOM KINGDOM Ashtead Group 1.26 UNITED Hikma Pharmaceuticals 0.22 UNITED Rolls-Royce Holdings 0.39 UNITED KINGDOM KINGDOM KINGDOM Associated British Foods 0.41 UNITED HSBC Hldgs 4.5 UNITED Royal Dutch Shell A 3.13 UNITED KINGDOM KINGDOM KINGDOM AstraZeneca 6.02 UNITED Imperial Brands 0.77 UNITED Royal Dutch Shell B 2.74 UNITED KINGDOM KINGDOM KINGDOM Auto Trader Group 0.32 UNITED Informa 0.4 UNITED Royal Mail 0.28 UNITED KINGDOM KINGDOM KINGDOM Avast 0.14 UNITED InterContinental Hotels Group 0.46 UNITED Sage Group 0.39 UNITED KINGDOM KINGDOM KINGDOM Aveva Group 0.23 UNITED Intermediate Capital Group 0.31 UNITED Sainsbury (J) 0.24 UNITED KINGDOM KINGDOM KINGDOM Aviva 0.84 UNITED International Consolidated Airlines 0.34 UNITED Schroders 0.21 UNITED KINGDOM Group KINGDOM KINGDOM B&M European Value Retail 0.27 UNITED Intertek Group 0.47 UNITED Scottish Mortgage Inv Tst 1 UNITED KINGDOM KINGDOM KINGDOM BAE Systems 0.89 UNITED ITV 0.25 UNITED Segro 0.69 UNITED KINGDOM -

BT Group Plc Annual Report 2020 BT Group Plc Annual Report 2020 Strategic Report 1
BT Group plc Group BT Annual Report 2020 Beyond Limits BT Group plc Annual Report 2020 BT Group plc Annual Report 2020 Strategic report 1 New BT Halo. ... of new products and services Contents Combining the We launched BT Halo, We’re best of 4G, 5G our best ever converged Strategic report connectivity package. and fibre. ... of flexible TV A message from our Chairman 2 A message from our Chief Executive 4 packages About BT 6 investing Our range of new flexible TV Executive Committee 8 packages aims to disrupt the Customers and markets 10 UK’s pay TV market and keep Regulatory update 12 pace with the rising tide of in the streamers. Our business model 14 Our strategy 16 Strategic progress 18 ... of next generation Our stakeholders 24 future... fibre broadband Culture and colleagues 30 We expect to invest around Introducing the Colleague Board 32 £12bn to connect 20m Section 172 statement 34 premises by mid-to-late-20s Non-financial information statement 35 if the conditions are right. Digital impact and sustainability 36 Our key performance indicators 40 Our performance as a sustainable and responsible business 42 ... of our Group performance 43 A letter from the Chair of Openreach 51 best-in-class How we manage risk 52 network ... to keep us all Our principal risks and uncertainties 53 5G makes a measurable connected Viability statement 64 difference to everyday During the pandemic, experiences and opens we’re helping those who up even more exciting need us the most. Corporate governance report 65 new experiences. Financial statements 117 .. -

The Second Release of Prima & Ultima
Notes to Editors: Recommended Retail Selling Price for the full set is £23,500 (including tax and duty) in UK Bottle sizes: 70cl Global Registration Live: 4th August 2021 Global Registration Closes: 23rd August 2021 Link for registration: www.theprimaandultimacollection.com The Second Release of Prima & Ultima: All of the Single Malt Scotch Whiskies included are natural cask strength, non-chill filtered, with no colour added. Auchroisk 1974 47-Year-Old | 48.7% ABV Bottled: 26.01.2121 | Single Refill European Oak Butt Number Bottled: 382 | Mulben, Speyside The very first cask to be filled at Auchroisk when distilling began on 15th January 1974, this cask has been kept back for many years for its character and its rarity. Remarkably rich and spicy, with fruity aromas and smooth flavours. Lagavulin 1992 28-Year-Old | 47.7% ABV Bottled: 20.01.2021 | 5 Freshly Charred American Oak Hogsheads Number Bottled: 1,081 | Port Ellen, Isle of Islay The first of its kind from a small experimental batch matured entirely on Islay in freshly charred American Oak hogsheads, this is a very rare Lagavulin. Time has mellowed the smoky nature of this spirit making it elegantly rounded and smooth in texture. Linkwood 1981 39-Year-Old | 52.9% ABV Bottled: 25.01.21 | 4 new American Oak casks, PX Oloroso seasoned Number Bottled: 701 | Elgin | Speyside Part of the first pioneering trial exploring different maturation processes at the distillery, this triple- rare expression gives it an incredible depth. The rich flavours and aromas of a PX/Oloroso-seasoned cask are deeply integrated with the creamy texture and spicy, toasted characters brought by the new American Oak. -
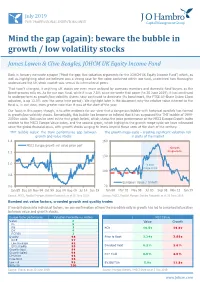
Mind the Gap (Again): Beware the Bubble in Growth / Low Volatility Stocks
July 2019 FOR PROFESSIONAL INVESTORS ONLY Mind the gap (again): beware the bubble in growth / low volatility stocks James Lowen & Clive Beagles, JOHCM UK Equity Income Fund Back in January we wrote a paper (‘Mind the gap: five valuation arguments for the JOHCM UK Equity Income Fund’) which, as well as highlighting what we believed was a strong case for the value contained within our fund, underlined how thoroughly undervalued the UK stock market was versus its international peers. That hasn’t changed, if anything UK stocks are even more unloved by overseas investors and domestic fund buyers as the Brexit process rolls on. As for our own fund, while it is up 7.8% since we wrote that paper (to 30 June 2019), it has continued to underperform as growth/low volatility shares have continued to dominate (its benchmark, the FTSE All-Share Index 12pm adjusted, is up 13.0% over the same time period). We highlight later in the document why the relative value inherent in the fund is, in our view, even greater now than it was at the start of the year. Our focus in this paper, though, is to offer evidence for our view that a dangerous bubble with historical parallels has formed in growth/low volatility stocks. Remarkably, this bubble has become so inflated that it has surpassed the TMT bubble of 1999- 2000 in scale. This can be seen in the first graph below, which shows the price performance of the MSCI Europe Growth index relative to the MSCI Europe Value index, and the second graph, which highlights the growth mega-cycle we have witnessed since the global financial crisis, with growth stocks surging to levels beyond those seen at the start of the century. -

Newton UK Income Pension PDF Factsheet
Adventurous 31 August 2021 Pension Fund SW Newton UK Income Pension (Series 4) With effect from 10 June 2019 the name of the manager of the underlying fund changed from This document is provided for the purpose of Newton to BNY Mellon. No other changes to the fund were made at this time and we will information only. This factsheet is intended for continue to refer to Newton in our literature. individuals who are familiar with investment terminology. Please contact your financial adviser if you need an explanation of the terms Asset Allocation (as at 31/07/2021) used. This material should not be relied upon United Kingdom Equity 85.1% as sufficient information to support an United States Equity 4.7% investment decision. The portfolio data on this factsheet is updated on a quarterly basis. France Equity 4.6% Switzerland Equity 4.5% Cash Equity 1.1% Fund Aim The fund aims for long-term total return from increasing levels of income from one year to the next along with long-term capital growth from the underlying assets, through investment solely in the Newton Higher Income OEIC fund. Basic Fund Information Sector Breakdown (as at 31/07/2021) Series 4 Unit Launch 22/08/2006 Consumer Staples 17.7% Date Consumer Discretionary 17.3% Fund Size £53.8m Financials 15.6% Sector ABI UK Equity Income Health Care 11.9% ISIN GB00B17M7F95 Industrials 11.3% MEX ID SWNHII Basic Materials 7.2% SEDOL B17M7F9 Energy 5.9% Manager Name Jon Bell, Ilga Haubelt Technology 4.8% Manager Since 25/09/2020, 25/09/2020 Utilities 4.6% Other 3.7% Top Ten Holdings (as at 31/07/2021) Regional Breakdown (as at 31/07/2021) UNILEVER PLC 5.6% GLAXOSMITHKLINE PLC 5.0% NATIONAL GRID PLC 4.6% DIAGEO PLC 4.5% BAE SYSTEMS PLC 3.9% BRITISH AMERICAN TOBACCO P.L.C. -

GEO Awards 2009.Pdf
Chief Executive Founding Corporate Sponsors Michael Bendorf, GEO Charles Schwab Board of Directors Morgan Stanley CHAIR: Carine M. Schneider, Global Shares Smith Barney VICE-CHAIR: Janet Cooper, Linklaters Compensation Phil Ainsley, Equiniti Venture Group John Bagdonas, Independent Computershare Juan Bonilla, Cuatrecasas Deutsche Bank June Anne Burke, Baker & McKenzie Alex Brown Lindsey Doud, RBC cees E*TRADE FINANCIAL Jay Foley, Morgan Stanley Smith Barney Global Reward Judith Greaves, Pinsent Masons Plan Group Véronique Japp, BNP Paribas Securities Services HBOS Employee Desiree Klein-Wagner, Allianz Equity Solutions Nancy Mesereau, Fidelity Stock Plan Services Hewitt Bacon Steve Morey, Proctor & Gamble & Woodrow Brian Ruff, Eli Lilly & Company Linklaters Mary Samsa, Seyfarth Shaw LLP Merrill Lynch Barbara Seta, UBS AG Pinsent Masons Sean Trotman, Deloitte PricewaterhouseCoopers James Wulforst, E*TRADE Financial Prudential Financial Corporate Services, Incorporated UBS Financial Advisory Council Services, Inc. CHAIR: Steve Morey, Proctor & Gamble Watson Wyatt Worldwide Karen Beyer, General Electric Provider Council Kelley Garrett, Microsoft Corp. Wendy Jennings, Riverbed Technology, Inc. CHAIR: Jewon Wee, ISP Advisors Yvonne Prang, McDonald’s Deutschland Inc. CO-CHAIR: Paul Stoddart, Brian Ruff, Eli Lilly & Company HBOS Employee Equity Bernice Toy, Cisco Systems, Inc. Solutions Nigel Turner, BP Debbie Veys, Vodafone Group Plc Dominique Welcomme, Vivendi 1442 E. Lincoln Ave. #487 Orange, CA 92865 US TEL +1 714-630-2908 • FAX +1 253-423-8390 • WEB www.globalequity.org ost Innovative M &CreativeD esign TheGEO war AJ� udges The 00 2 9 war ACategories� The 2 009 A war� Categories A�out �he GEO A war�s Welcome �o �heGEO 2009 war s Dear GEO Members, A � The 2009 GEO Awards celebrate outstanding performance in the international employee share plan commu- nity by honoring the impressive achievements of ten companies from around the world. -

Annual Report and Accounts 2020 and Is an Exact Copy of the Printed Document Provided to Unilever’S Shareholders
Disclaimer This is a PDF version of the Unilever Annual Report and Accounts 2020 and is an exact copy of the printed document provided to Unilever’s shareholders. Certain sections of the Unilever Annual Report and Accounts 2020 have been audited. These are on pages 112 to 167, and those parts noted as audited within the Directors’ Remuneration Report on pages 90 to 99. The maintenance and integrity of the Unilever website is the responsibility of the Directors; the work carried out by the auditors does not involve consideration of these matters. Accordingly, the auditors accept no responsibility for any changes that may have occurred to the financial statements since they were initially placed on the website. Legislation in the United Kingdom and the Netherlands governing the preparation and dissemination of financial statements may differ from legislation in other jurisdictions. Except where you are a shareholder, this material is provided for information purposes only and is not, in particular, intended to confer any legal rights on you. This Annual Report and Accounts does not constitute an invitation to invest in Unilever shares. Any decisions you make in reliance on this information are solely your responsibility. The information is given as of the dates specified, is not updated, and any forward-looking statements are made subject to the reservations specified in the cautionary statement on the inside back cover of this PDF. Unilever accepts no responsibility for any information on other websites that may be accessed from this site -

Unilever Annual Report on Form 20-F 2020 in This Report
Disclaimer This is a PDF version of the Annual Report on Form 20-F 2020 and is an exact copy of the document filed with the SEC at www.sec.gov. Certain sections of the Annual Report on Form 20-F 2020 have been audited. These are on pages 112 to 167. The maintenance and integrity of the Unilever website is the responsibility of the Directors; the work carried out by the auditors does not involve consideration of these matters. Accordingly, the auditors accept no responsibility for any changes that may have occurred to the financial statements since they were initially placed on the website. Legislation in the United Kingdom and the Netherlands governing the preparation and dissemination of financial statements may differ from legislation in other jurisdictions. Except where you are a shareholder, this material is provided for information purposes only and is not, in particular, intended to confer any legal rights on you. This Annual Report on Form 20-F does not constitute an invitation to invest in Unilever shares. Any decisions you make in reliance on this information are solely your responsibility. The information is given as of the dates specified, is not updated, and any forward-looking statements are made subject to the reservations specified in the cautionary statement on the inside back cover of the Annual Report on Form 20-F 2020. Unilever accepts no responsibility for any information on other websites that may be accessed from this site by hyperlinks. Purpose-led, future-fit Unilever Annual Report on Form 20-F 2020 In this report -

Annual Report and Accounts 06
Annual Report and Accounts 06 Annual Review and Summary Financial Statement and Directors’ Report and Accounts 2006 WorldReginfo - 98675577-a5f8-41aa-ac01-37c1b8bcb2da About this combined Report The format of our reporting has been changed to accommodate the inclusion, for the first time, of an Operating and Financial Review (OFR). In preparing the OFR, we have sought to take into account, where considered appropriate, the best practice set out in the UK Accounting Standards Board’s ‘Reporting Statement: Operating and Financial Review’. We have responded to the spirit of the OFR by offering shareholders a balanced and comprehensive analysis of our current business and describing the significant industry trends that are likely to influence our future prospects. For the first time, we have published our Key Performance Indicators, some other important Business Measures and the Group’s Key Risk Factors. We have also attempted to avoid having too much information in one publication. Our corporate website bat.com has a wealth of material about the Group and, in May 2007, we plan to publish our latest Social Report, detailing progress during 2006 on a range of commitments and actions. We will carefully consider the structure and content of our future reporting in the light of developments in the field and the advice and comments we receive about this publication. The full content of this combined Report is under this flap. Leave it open as a reference to find your way to the different topics covered. Cautionary Statement: the Operating and Financial Review and certain other sections of this document contain forward looking statements which are subject to risk factors associated with, among other things, the economic and business circumstances occurring from time to time in the countries and markets in which the Group operates.